Published online Mar 21, 2012. doi: 10.3748/wjg.v18.i11.1208
Revised: April 26, 2011
Accepted: May 3, 2011
Published online: March 21, 2012
AIM: To determine the expression of HER2 and bradykinin B1 receptors (B1R) in the two pathogenic models of gallbladder cancer: the metaplasia-dysplasia-carcinoma and the adenoma-carcinoma pathways.
METHODS: Receptor proteins were visualized by immunohistochemistry on 5-μm sections of paraffin-embedded tissue. Expression of both receptors was studied in biopsy samples from 92 patients (6 males and 86 females; age ranging from 28 to 86 years, mean 56 years). High HER2 expression in specimens was additionally investigated by fluorescence in situ hybridization. Cell proliferation in each sample was assessed by using the Ki-67 proliferation marker.
RESULTS: HER2 receptor protein was absent in adenomas and in normal gallbladder epithelium. On the contrary, there was intense staining for HER2 on the basolateral membrane of epithelial cells of intestinal metaplasia (22/24; 91.7%) and carcinoma in situ (9/10; 90%), the lesions that displayed a significantly high proliferation index. Protein up-regulation of HER2 in the epithelium with metaplasia or carcinoma in situ was not accompanied by HER2 gene amplification. A similar result was observed in invasive carcinomas (0/12). The B1R distribution pattern mirrored that of HER2 except that B1R was additionally observed in the adenomas. The B1R appeared either as cytoplasmic dots or labeling on the apical cell membrane of the cells composing the epithelia with intestinal metaplasia (24/24; 100%) and carcinoma in situ (10/10; 100%) and in the epithelial cells of adenomas. In contrast, both HER2 (4/12; 33%) and B1R (1/12; 8.3%) showed a low expression in invasive gallbladder carcinomas.
CONCLUSION: The up-regulation of HER2 and B1R in precursor lesions of gallbladder carcinoma suggests cross-talk between these two receptors that may be of importance in the modulation of cell proliferation in gallbladder carcinogenesis.
- Citation: Toledo C, Matus CE, Barraza X, Arroyo P, Ehrenfeld P, Figueroa CD, Bhoola KD, del Pozo M, Poblete MT. Expression of HER2 and bradykinin B1 receptors in precursor lesions of gallbladder carcinoma. World J Gastroenterol 2012; 18(11): 1208-1215
- URL: https://www.wjgnet.com/1007-9327/full/v18/i11/1208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i11.1208
Gallbladder carcinoma is an aggressive cancer of the gastrointestinal tract with incidence three times greater in the female population of Chile, India and Japan when compared to men[1,2]. The most common type of gallbladder cancer is adenocarcinoma. However, early diagnosis is difficult because most of the cases are detected only at an advanced stage following laparotomy.
Successive bouts of inflammation cause continuous and long lasting damage to the gallbladder epithelium, which thereby transforms into an architecturally abnormal regenerative epithelium very similar to low grade dysplasia. Injury and repair are also related to well known metaplastic changes of the mucosa, such as pyloric and intestinal metaplasia, and to other rare types of metaplasia[3]. Intestinal metaplasia usually develops into dysplasia and is frequently observed adjacent to carcinoma. Pyloric and intestinal metaplasias are associated with gallbladder carcinoma, but intestinal metaplasia show a greater relationship with malignancy[4,5]. Analysis of serial sections indicates the presence of microinvasion foci in the lamina propria next to carcinoma in situ and also that dysplasia and carcinoma in situ occur close to areas of intact mucosa in nearly all invasive carcinomas[6].
Among the pathogenic models that explain the neoplastic transformation of gallbladder epithelium are the metaplasia-dysplasia-carcinoma and the adenoma-carcinoma pathways[5]. Morphological and molecular studies have shown that these two entities correspond to independent biological events. Therefore, we have used representative gallbladder samples of both putative pathways to investigate the expression of two receptor molecules involved in cell proliferation, namely the HER2 and bradykinin B1 receptors (B1R). The HER2 (c-erbB-2) receptor is recognized to be of clinical importance because of its prognostic value in determining progression of some types of breast tumors. Similarly, B1R has recently been shown to induce the proliferation of estrogen-sensitive breast cancer cells by turning on the transactivation of the epidermal growth factor receptor (EGFR), a signal-transduction pathway involved also in the activation of HER2[7]. As with breast cancer, expression of HER2 in gallbladder carcinoma has been associated with progression of malignancy and linked to poor patient survival[8-11]. Furthermore, constitutive expression of HER2 in transgenic mice causes adenocarcinoma of the gallbladder[12] suggesting a key role for this member of the EGFR family in such neoplasia. Although the expression levels of HER2 have been previously investigated in invasive gallbladder cancer[13], so far no studies appear to have examined its expression in the putative precursor lesions of gallbladder carcinoma.
The B1R belongs to the family of G protein-coupled rhodopsin-like receptors which, upon stimulation by analogues that lack the Arg9 from the carboxy terminus of the bradykinin molecule, trigger several second messenger signaling systems that control cell differentiation, proliferation and/or migration[14,15]. The kinin B1R agonists belong to a family of bioactive peptides produced locally and with paracrine activity, that are formed from precursor molecules by the proteolytic action of enzymes called kallikreins (kininogenases)[14,15]. So far, only a few studies have evaluated the role of B1R as well as the underlying molecular mechanisms that trigger its activation in cancer cells. Recent reports have suggested that the B1R is an important player in lung, prostate and breast cancer by regulating tumoral growth, migration and invasion[7,16,17]. In addition, some of the cellular actions of B1R stimulation are a consequence of EGFR transactivation[7]. However, the status of B1R in other neoplastic disorders such as gallbladder carcinoma has not been investigated previously. Thus, the primary aim of our study was to perform a comprehensive evaluation of the expression values for HER2 and B1R in the metaplasia-dysplasia-carcinoma and adenoma-carcinoma pathways.
This study was performed in accordance with the Declaration of Helsinki of the World Medical Association. The designated experiments were approved by the Ethical Committees of Hospital Base Valdivia, Universidad Austral de Chile and the National Fund for Development of Science and Technology in Chile (FONDECYT) that included guidelines for the protection of human subjects.
The study was performed on 92 routinely resected gallbladders retrieved from the surgical pathology archive of the Servicio de Patologia, Hospital Base Valdivia, Chile. Of the 92 specimens, 6 were from males, and 86 were from females; the patients ranged in age from 28 to 86 years (mean, 56 years). The data on age, sex of patients bearing adenomas, gallbladder adenocarcinomas and their putative precursor lesions are summarized in Table 1. The following categories were recorded: (1) normal mucosa (n = 5), gallbladders that were resected due to lithiasis and elective surgery; (2) pyloric-type adenoma (n = 15); (3) intestinal-type adenoma (n = 6); (4) pyloric metaplasia (n = 20); (5) intestinal metaplasia (n = 24); (6) carcinoma in situ (n = 10); and (7) invasive carcinoma (n = 12) (Table 1). Invasive adenocarcinomas examined histologically were classified into well-differentiated with a papillary pattern of growth (8 cases), poorly differentiated (2 cases), mucinous well-differentiated (1 case) and signet ring (1 case) adenocarcinomas.
| Lesion | Sex | Age (yr, mean ± SD) |
| Normal mucosa (n = 5) | F (5) | 54.6 ± 9.0 |
| Pyloric adenoma (n = 15) | F (13)/M (2) | 55.2 ± 3.5 |
| Intestinal adenoma (n = 6) | F (6) | 68.5 ± 1.7 |
| Pyloric-type metaplasia (n = 20) | F (20) | 51.5 ± 3.2 |
| Intestinal metaplasia (n = 24) | F (24) | 50.0 ± 3.4 |
| Carcinoma in situ (n = 10) | F (9)/M (1) | 65.8 ± 2.5 |
| Invasive carcinoma (n = 12) | F (9)/M (3) | 66.0 ± 3.8 |
Each gallbladder was fixed in 10% buffered formalin and embedded in paraffin according to conventional protocols. Sections from each block were stained with hematoxylin and eosin for precise histopathological classification before immunostaining. In all cases, two to four blocks were used for immunohistochemical evaluation.
This method was performed as previously described by Molina et al[7]. Briefly, paraffin sections were incubated with primary antibodies overnight at 22 °C in a water bath that was used as a moist chamber. The primary antibody sources, dilutions, antigen retrieval and incubation conditions are listed in Table 2. Bound primary antibodies were localized by the biotin/streptavidin immunoperoxidase technique using the LSAB+ kit (Dako, Carpinteria, CA, United States). Peroxidase was developed with diaminobenzidine and hydrogen peroxide. Sections were counterstained with Harris hematoxylin. Negative controls included omission of primary antibody and its replacement by non-immune immunoglobulins of the same species or isotype matched immunoglobulins under identical conditions.
| Antibody | Source | Dilution/incubation | Microwave antigen retrieval |
| HER2 | Novocastra, NCL-CBE-356 | 1:200/overnight | No |
| Bradykinin B1R | Santa Cruz, sc-25484 | 1:300/overnight | 90 °C/7 min, Tris-HCl buffer pH 10 |
| Merck | 1:500/overnight | No | |
| Ki-67 | Dako, A0047 | 1:200/overnight | 90 °C/15 min, Citrate buffer pH 6 |
Immunolabeling was scored as positive (presence of staining) or negative (absence of staining) and then expressed as percentage of total number of normal mucosa, metaplasia, carcinoma in situ and invasive carcinoma cases examined. Staining was considered positive when over 30% of the gallbladder epithelium was immunostained. Only cell membrane immunoreactivity was considered positive for HER2 staining. Cell proliferation index was determined by recording the immunolabeled Ki-67 antigen in gallbladder carcinoma and its putative precursor lesions. For this purpose, nuclei were counted at x40 magnification in three different fields selected at random. The procedure was repeated twice by two independent observers for each lesion and the counts were averaged. The Ki-67 cell proliferation index was derived by dividing the average Ki-67 count by the average total number of nuclei in one field.
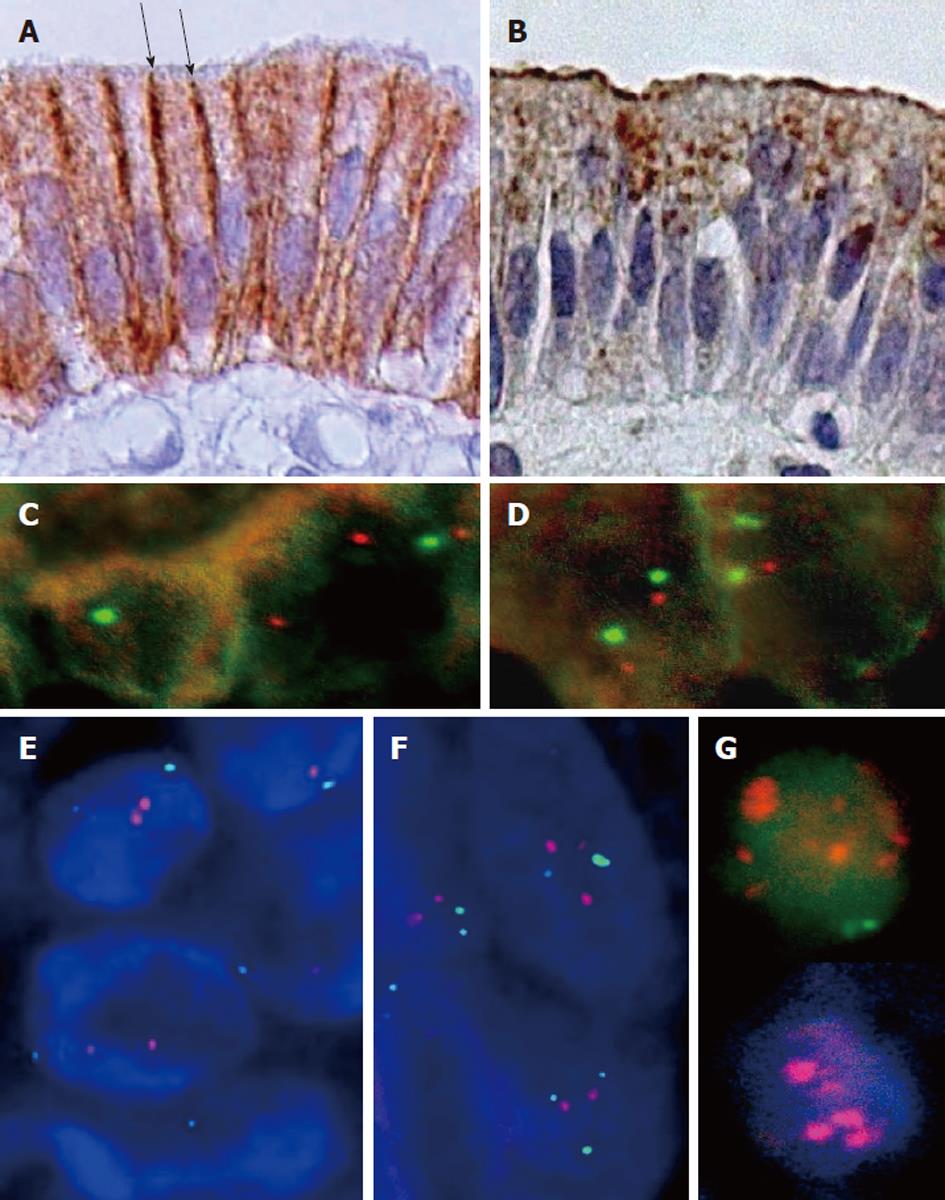
Fluorescence in situ hybridization (FISH) technique for HER2 was performed on 3-μm thick tissue sections according to the protocol of HER2 FISH PharmDx™ Kit (Dako). Hybridization was performed with a mixture of HER2-Texas Red and cen-17 labeled with fluorescein isothiocyanate. A breast cancer sample classified as HER2 (+3) was used as positive control. For each condition at least two samples were analyzed using a fluorescence Nikon Labophot-2, and at least 50 nuclei were visualized in each sample.
Differences in the occurrence of immunolabeling were evaluated using Fischer’s exact probability test with the aid of the software JMP Statistical Discovery Software 8.0 (SAS Institute, Cary, NC, United States). Probability values less than 0.05 were considered to be statistically significant.
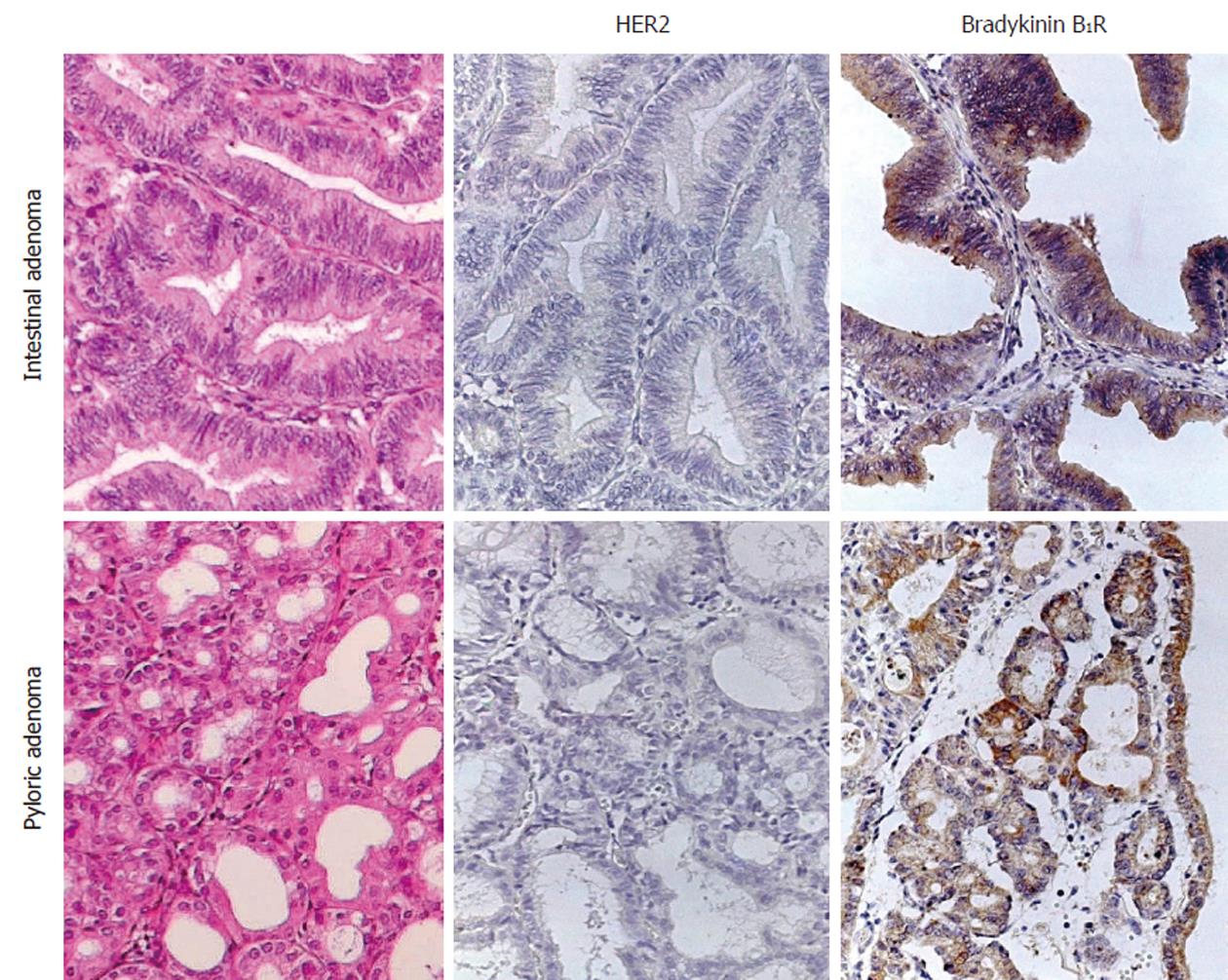
Normal, non-metaplastic epithelium was comprised of regular segments of tall columnar cells with basal nuclei and sporadic small apical vacuoles. Pyloric-type adenomas showed their typical morphology that was comprised of tightly packed pyloric or antral-type mucous glands similar to mucous glands of the stomach (Figure 1). Intestinal-type adenomas consisted of papillary structures lined by columnar epithelium with elongated and pseudostratified nuclei (Figure 1). Pyloric-type metaplasia was distinguished by glands lined by columnar cells with vacuolated cytoplasm and flattened nuclei located basally; the glands formed small lobular structures throughout the lamina propria. Epithelium with intestinal metaplasia was composed of tall columnar cells with a brush border and variable proportions of goblet cells. This type of metaplasia was often seen adjacent to invasive adenocarcinoma. Carcinoma in situ was characterized by the presence of epithelial cells that showed frequent mitosis and marked alterations in the size and shape of the nuclei such as hypercromasia, overlapping and crowding.
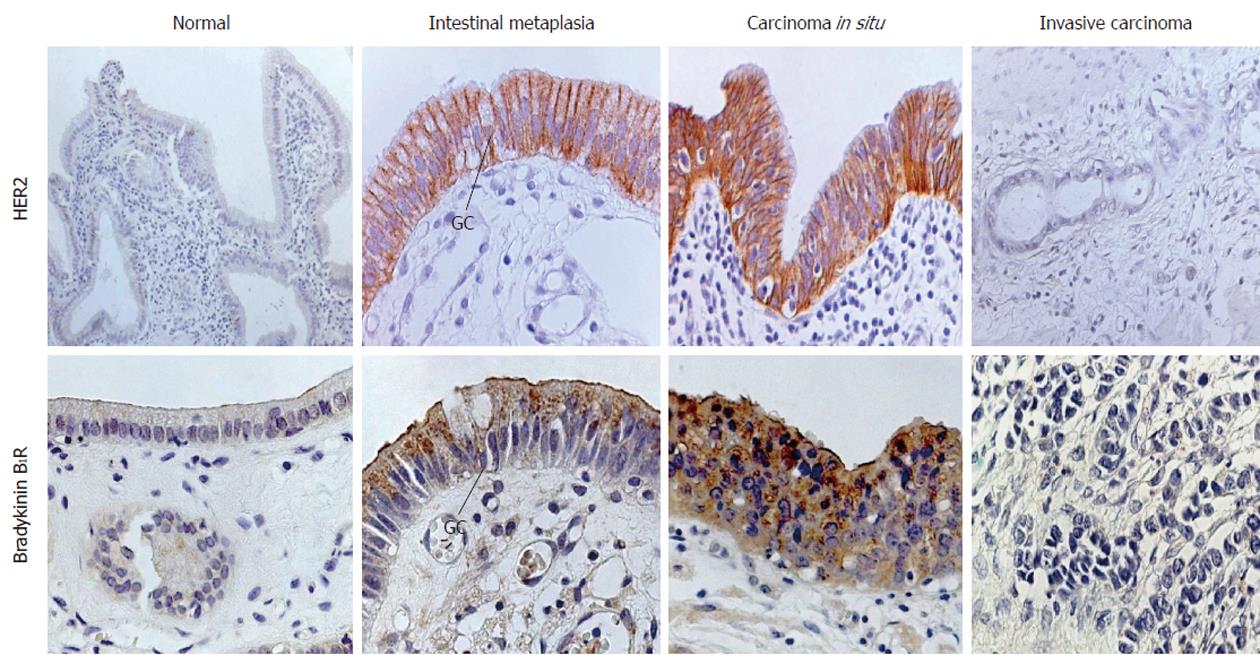
No immunolabeled HER2 receptor was visualized in normal gallbladder epithelium and no significant expression of the protein was visualized in adenomas of either pyloric- or intestinal-type (Table 3; Figures 1 and 2). In contrast, an intense immunoreactivity was observed in the epithelium with intestinal metaplasia (22/24) and carcinoma in situ (9/10), with decreased staining in invasive carcinomas (4/12) (Table 3; Figure 2) and none in pyloric metaplasia and non-epithelial tissues (not shown). The HER2 immunolabeling was confined to the basolateral cell membrane region of all metaplastic epithelial cells, whereas the luminal membrane was devoid of staining (Figures 2 and 3). At a higher magnification, the boundary between the two segments was clearly delineated by the different immunoreactivity of the cells in the apical membrane when compared to those of the basolateral cell membrane (Figure 3). Loss of polarity in the epithelia with carcinoma in situ resulted in a complete membrane staining of some of the neoplastic epithelial cells. With FISH staining, HER2 gene appeared as two red signals in normal and metaplastic epithelia and two to four signals in carcinoma in situ and invasive carcinoma, accompanied by the corresponding green signals pointing out the chromosome 17 centromeres (HER2/CEN-17 < 1.8) that indicated lack of amplification (Figure 3C-F). On the contrary, a clear amplification of HER2 gene was observed in HER2 (+3) breast cancer samples that were used as positive controls (Figure 3G).
| Receptor/marker | Normal epithelium | Pyloric adenoma | Intestinal adenoma | Intestinal metaplasia | Carcinoma in situ | Invasive carcinoma |
| HER2 | 0/5 | 0/14 | 1/6 (17) | 22/24 (91.7)a | 9/10 (90)a | 4/12 (33) |
| Bradykinin B1R | 0/5 | 10/15 (67)a | 6/6 (100)a | 24/24 (100)a | 10/10 (100)a | 1/12 (8.3) |
| Ki-671 | 0.05 ± 0.005 (1.1) | 0.05 ± 0.01 (5.1) | 0.10 ± 0.01 (10.8) | 0.36 ± 0.03b (36.7) | 0.37 ± 0.05b (37.5) | 0.42 ± 0.03b (42) |
The B1R protein also followed a cell membrane distribution pattern. Immunolabeled B1R was visualized only in one case of the invasive carcinomas (Table 3). In contrast, samples with intestinal metaplasia and carcinoma in situ showed an intense staining for B1R that appeared as cytoplasmic dots or labeling in the apical cell membrane region of metaplastic cells and in the epithelium with carcinoma in situ (Figures 2 and 3). Furthermore, B1R immunolabeling was observed in epithelial cells of pyloric- and intestinal-type adenomas, including the surface epithelium of adenomas (Figure 1 and Table 3).
By using Ki-67, we confirmed that the observed cell proliferation was significantly higher in epithelia with metaplasia, carcinoma in situ and in cells of invasive gallbladder carcinomas than in normal epithelium and in epithelial cells of both pyloric- and intestinal-type adenomas (Table 3).
Two pathogenic models designated as adenoma-carcinoma and metaplasia-dysplasia-carcinoma have been used to explain neoplastic transformation of the gallbladder epithelium. However, intestinal metaplasia is considered as the major precursor lesion that later progresses into carcinoma in situ and invasive adenocarcinoma. The question of whether expression of receptors such as HER2, a tyrosine kinase orphan receptor, and the B1R, a G protein-coupled receptor, are enhanced in such carcinogenic changes of the gallbladder epithelium formed the focus of the current study[7,15,16,18,19].
Remarkably, the expression of HER2 and bradykinin B1R followed a similar pattern of distribution in invasive carcinoma and in its putative precursor lesions. Both receptors were absent in the normal epithelium but were strongly expressed in carcinoma in situ and the epithelia with intestinal metaplasia. Absence of HER2 staining in normal gallbladder epithelium is in agreement with previous studies performed using normal breast tissue[20]. Two previous reports failed to demonstrate expression of HER2 protein in gallbladder dysplasia[13,21]. The discrepancy between these findings and our results may be explained by the use of different immunostaining procedures (e.g., monoclonal vs polyclonal antibodies and sensitivity of the technique), time of fixation, preservation of antigenic sites and number of samples analyzed. In our study, HER2 receptor protein stained intensely along the basolateral plasma membrane of the metaplastic and carcinoma in situ cells. A similar pattern of staining has been observed in apocrine metaplasia of the breast where HER2 immunoreactivity appeared restricted to the basolateral plasma membrane of the metaplastic cells[22]. It is well known that tight junctions morphologically divide cell membranes of non-neoplastic polarized epithelial cells into two regions: an apical one which faces the lumen and often has specialized features such as cilia or a brush border of microvilli; and a basolateral region, which covers the rest of the cell[23]. Further, tight junctions prevent proteins and lipids from diffusing between the basolateral and apical regions, so that not only the protein but also the lipid composition of the two membrane regions is different[23]. Therefore, the HER2 immunoreactivity, observed in the basolateral cell membrane domain of metaplastic gallbladder epithelium, is not comparable to that scored by the Hercep TestTM staining protocol, which is based on the immunoreactivity present on the cell membrane of invasive breast cancer cells that are not supported by a basement membrane, i.e., neoplastic non-polarized cells. Despite over-expression of HER2 receptor protein in metaplastic epithelia and carcinoma in situ, our results using FISH revealed that this event was not accompanied by HER2 gene amplification suggesting that this change may not be relevant for gallbladder cancer. Studies using FISH, but restricted to invasive carcinomas of the gallbladder, have shown amplification of the HER2 gene only in approximately 10% of the cases investigated[8]. Absence of HER2 gene amplification in our tissue samples with invasive carcinoma may be due to our low number of cases compared with other published studies[8]. Some authors suggest that HER2 over-expression is due to gene deregulation rather than gene amplification because in some reports there is no strict correlation between receptor protein expression and gene amplification[24]. The HER2 receptor seems to be a key player since its constitutive expression leads to the development of gallbladder adenocarcinoma in 100% of transgenic mice[12]. Moreover, increased HER2/EGFR (erbB-1) heterodimer formation, hyperphosphorylation of tyrosine residues of both HER2 and EGFR (but not erbB-3 or erbB-4) and activation of the mitogen-activated protein kinases (MAPK) signaling pathway were observed in the gallbladder epithelium of the transgenic mice[12]. Over-expression of the HER2 receptor protein, detected immunohistochemically in nearly all breast specimens showing carcinoma in situ of high grade, has been interpreted as an expression of rapid growth since its presence is related to cellular proliferation[25,26]. Our results show that the epithelia with intestinal metaplasia and carcinoma in situ display the higher values for Ki-67 cell proliferation marker. The fact that the HER2 gene shows amplification only in 10% of the invasive gallbladder carcinomas[8] contrasts with the high levels of protein expression observed by us in intestinal metaplasia and carcinoma in situ, and suggests that major activity of HER2 may occur in these precursor lesions. Further, the expression of HER2 in other invasive tumors of the gastrointestinal tract such as gastric cancer has revealed that less than 10% of all invasive tumors showed HER2 expression and that there is no relationship between its expression, patient survival or TNM stage[27].
Members of the EGFR family are activated not only by direct binding of their corresponding ligands but also by transactivation triggered after stimulation of G protein-coupled receptors such as the B1R[15,19]. We have recently reported B1R binding sites in breast carcinoma and showed that B1R stimulation induces the proliferation of MCF-7 and ZR-75 breast cancer cells, an effect that depends on the transactivation of the EGFR and the subsequent activation of the MAPK signaling pathway[7]. Moreover, stimulation of the B1R increases the release of metalloproteases-2 and -9 from breast cancer cells[28]. Both metalloproteases are considered key enzymes for tumor invasion and metastasis, because they have the capacity to degrade type IV collagen, the major protein component of basement membranes. Here, we have described, for the first time, the expression of B1R in precursor lesions of gallbladder carcinomas (i.e., intestinal metaplasia and carcinoma in situ), conditions in which its expression is maximal and associated with high proliferation rates of the epithelial cells.
The kinin B1R agonists are short-lived peptides that exert most of their actions in a paracrine fashion for which the presence of kallikreins, kinin precursors and expression of the appropriate receptor is required[14]. In a previous report, we showed that both tissue kallikrein and the kinin precursors are present in the human gallbladder suggesting that kinin formation is feasible in this tissue[29]. Tissue kallikrein (KLK1/hK1, kininogenase) is expressed by gallbladder epithelial cells whereas the kininogens, substrates of hK1, diffuse from submucosal blood vessels to fill the interstitial space between epithelial cells[29]. A further source of tissue kallikrein is the neutrophil that infiltrates the inflamed gallbladder during acute episodes of cholecystitis and thereby releases hK1 in the vicinity of epithelial cells[29]. Additionally, the natural B1R agonist induces migration of glioma cells via up-regulation of cyclooxygenase-2 expression[30]; whether the B1R produces a similar effect in gallbladder cancer cells remains to be investigated using gallbladder cancer cell lines.
The identical pattern of expression observed for B1 and HER2 receptors in precursor lesions of gallbladder carcinoma suggests a cooperative relationship, orientated towards promoting functional cell signaling that may result in an increased proliferation of the gallbladder epithelium.
The authors (Poblete MT and Figueroa CD) wish to record our deepest gratitude and appreciation for the conceptual support and advice given during our entire career by Drs Norambuena L and Caorsi I. We also wish to thank Ms Johana Mejia for her assistance with statistical procedures and Merck and Co Inc, West Point, PA, United States for the kind gift of anti-B1 receptor antibody.
Gallbladder carcinoma is an aggressive malignancy of the gastrointestinal tract, and its early diagnosis is difficult, because most of the cases are detected at an advanced stage at laparotomy. It is well known that the HER2 receptor is involved in the carcinogenesis of many malignancies. Further, recent reports show that the bradykinin B1 receptor (B1R) stimulates the proliferation of breast cancer cells. It is not known whether HER2 and B1R are overexpressed in precursor lesions of gallbladder carcinoma and whether they serve as biomarkers for early neoplastic transformation of gallbladder epithelium.
Most studies related to HER2 and gallbladder cancer have been focused on advanced stages of the disease, but not in their precursor lesions. So far, there have been no investigations on the expression levels of B1R in this neoplasm. Because HER2 has a low expression in invasive gallbladder cancer, it has been proposed by some authors that it is not relevant to gallbladder carcinoma.
The novel finding of high expression of HER2 and B1R in precursor lesions of gallbladder carcinoma suggests that they enhance cell proliferation and play a significant role in gallbladder carcinogenesis.
Because of the high HER2 and B1R expression data in precursor lesions of gallbladder carcinoma, and the therapeutic efficacy of anti-HER2 treatments in breast tumors, future studies should focus on HER2 and B1R as biomarkers for the detection of early neoplastic transformations in the gallbladder epithelium.
The HER2 is a tyrosine kinase receptor, member of the epidermal growth factor receptor family. Growth factors bind to receptors and initiate cell proliferation, migration, invasion, resistance to apoptosis and angiogenesis. B1R belongs to the family of G protein-coupled receptors, which upon stimulation by analogues trigger cell proliferation and secretion of metalloproteases in cancer cells: proteases that assist cancer cells to invade normal human tissue. The results clearly indicate that both HER2 and the B1R play a significant role in transformation of epithelial cells through the metaplasia-dysplasia-carcinoma pathway.
This article has very well explored the HER2 and B1R in gallbladder carcinoma. While HER2 and B1R are scarcely expressed in invasive gallbladder carcinoma, their expression is upregulated in precursor lesions of this neoplasm which may help in early diagnosis and also provide some pathways of carcinogenesis. This study is worthy.
Peer reviewers: Yoshiaki Murakami, MD, Division of Clinical Medical Science, Department of Surgery, Graduate School of Biomedical Science, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan; Gopal Nath, MD, PhD, Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, India
S- Editor Zhang DN L- Editor Logan S E- Editor Zheng XM
| 1. | Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 572] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 2. | Medina E, Kaempffer AM. [Cancer mortality in Chile: epidemiological considerations]. Rev Med Chil. 2001;129:1195-1202. [PubMed] |
| 3. | Murakata LA, Albores-Saavedra J. Benign and malignant tumors of the gallbladder and extrahepatic biliary tract. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Philadelphia: Saunders 2004; 639-672. |
| 4. | Duarte I, Llanos O, Domke H, Harz C, Valdivieso V. Metaplasia and precursor lesions of gallbladder carcinoma. Frequency, distribution, and probability of detection in routine histologic samples. Cancer. 1993;72:1878-1884. [PubMed] |
| 5. | Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Albores-Saavedra J, Henson DE. Gallbladder, extrahepatic bile ducts, and ampulla of Vater. Pathology of incipient neoplasia. Hong Kong: Oxford University Press Inc 2001; 263-280. |
| 7. | Molina L, Matus CE, Astroza A, Pavicic F, Tapia E, Toledo C, Perez JA, Nualart F, Gonzalez CB, Burgos RA. Stimulation of the bradykinin B(1) receptor induces the proliferation of estrogen-sensitive breast cancer cells and activates the ERK1/2 signaling pathway. Breast Cancer Res Treat. 2009;118:499-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Kalekou H, Miliaras D. Immunohistochemical study of microvessel density, CD44 (standard form), p53 protein and c-erbB2 in gallbladder carcinoma. J Gastroenterol Hepatol. 2004;19:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in Indian patients manuscript id: 8962091628764582. BMC Cancer. 2006;6:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kim YW, Huh SH, Park YK, Yoon TY, Lee SM, Hong SH. Expression of the c-erb-B2 and p53 protein in gallbladder carcinomas. Oncol Rep. 2001;8:1127-1132. [PubMed] |
| 12. | Kiguchi K, Carbajal S, Chan K, Beltrán L, Ruffino L, Shen J, Matsumoto T, Yoshimi N, DiGiovanni J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971-6976. [PubMed] |
| 13. | Matsuyama S, Kitajima Y, Sumi K, Mori D, Satoh T, Miyazaki K. Gallbladder cancers rarely overexpress HER-2/neu, demonstrated by Hercep test. Oncol Rep. 2004;11:815-819. [PubMed] |
| 14. | Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1-80. [PubMed] |
| 15. | Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 744] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 16. | Taub JS, Guo R, Leeb-Lundberg LM, Madden JF, Daaka Y. Bradykinin receptor subtype 1 expression and function in prostate cancer. Cancer Res. 2003;63:2037-2041. [PubMed] |
| 17. | Gera L, Stewart JM, Fortin JP, Morissette G, Marceau F. Structural modification of the highly potent peptide bradykinin B1 receptor antagonist B9958. Int Immunopharmacol. 2008;8:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Esseghir S, Reis-Filho JS, Kennedy A, James M, O'Hare MJ, Jeffery R, Poulsom R, Isacke CM. Identification of transmembrane proteins as potential prognostic markers and therapeutic targets in breast cancer by a screen for signal sequence encoding transcripts. J Pathol. 2006;210:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Matus CE, Ehrenfeld P, Pavicic F, Sarmiento JM, Astroza A, Sanchez T, Salem C, Concha M, Vidal MA, Gonzalez CB. Activation of kinin B receptor triggers differentiation of cultured human keratinocytes. Br J Dermatol. 2008;159:792-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Pavelic ZP, Pavelic L, Lower EE, Gapany M, Gapany S, Barker EA, Preisler HD. c-myc, c-erbB-2, and Ki-67 expression in normal breast tissue and in invasive and noninvasive breast carcinoma. Cancer Res. 1992;52:2597-2602. [PubMed] |
| 21. | Kamel D, Pääkkö P, Nuorva K, Vähäkangas K, Soini Y. p53 and c-erbB-2 protein expression in adenocarcinomas and epithelial dysplasias of the gall bladder. J Pathol. 1993;170:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Selim AG, El-Ayat G, Wells CA. Expression of c-erbB2, p53, Bcl-2, Bax, c-myc and Ki-67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch. 2002;441:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Walter P. Vesicular traffic in the secretory and endocytic pathways. Molecular biology of the cell. 2nd ed. New York and London: Garland Publishing Inc 1994; 599-651. |
| 24. | Ukita Y, Kato M, Terada T. Gene amplification and mRNA and protein overexpression of c-erbB-2 (HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by fluorescence in situ hybridization, in situ hybridization, and immunohistochemistry. J Hepatol. 2002;36:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | van de Vijver MJ, Peterse JL, Mooi WJ, Wisman P, Lomans J, Dalesio O, Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 586] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Allred DC, O'Connell P, Fuqua SA, Osborne CK. Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat. 1994;32:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [PubMed] |
| 28. | Ehrenfeld P, Conejeros I, Pavicic MF, Matus CE, Gonzalez CB, Quest AF, Bhoola KD, Poblete MT, Burgos RA, Figueroa CD. Activation of kinin B1 receptor increases the release of metalloproteases-2 and -9 from both estrogen-sensitive and -insensitive breast cancer cells. Cancer Lett. 2011;301:106-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Trevisani M, Amadesi S, Schmidlin F, Poblete MT, Bardella E, Maggiore B, Harrison S, Figueroa CD, Tognetto M, Navarra G. Bradykinin B2 receptors mediate contraction in the normal and inflamed human gallbladder in vitro. Gastroenterology. 2003;125:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Lu DY, Leung YM, Huang SM, Wong KL. Bradykinin-induced cell migration and COX-2 production mediated by the bradykinin B1 receptor in glioma cells. J Cell Biochem. 2010;110:141-150. [PubMed] |