Published online Jan 7, 2012. doi: 10.3748/wjg.v18.i1.44
Revised: July 6, 2011
Accepted: July 11, 2011
Published online: January 7, 2012
AIM: To investigate the effects of proton pump inhibitor (PPI) treatment on stool antigen test using the TestMate pylori enzyme immunoassay.
METHODS: This study assessed 28 patients [16 men and 12 women; mean age (63.1 ± 5.9) years; range, 25-84 years] who underwent stool antigen test and urea breath test (UBT) before and after PPI administration.
RESULTS: Using the UBT as the standard, the sensitivity, specificity and agreement of the stool antigen test in all 28 patients were 95.2%, 71.4%, and 89.3%, respectively, before PPI administration, and 88.9%, 90.9%, and 89.3%, respectively, after PPI treatment. Mean UBT values were 23.98% ± 5.33% before and 16.19% ± 4.75% after PPI treatment and, in 15 patients treated for ≥ 4 wk, were significantly lower after than before 4 wk of PPI treatment (12.58% ± 4.49% vs 24.53% ± 8.53%, P = 0.048). The mean optical density (A450/630) ratios on the stool antigen test were 1.16 ± 0.20 before and 1.17 ± 0.24 after PPI treatment (P = 0.989), and were 1.02 ± 0.26 and 0.69 ± 0.28, respectively, in the group treated for > 4 wk (P = 0.099).
CONCLUSION: The stool antigen test was equally sensitive to the UBT, making it a useful and reliable diagnostic method, even during PPI administration.
-
Citation: Kodama M, Murakami K, Okimoto T, Fukuda Y, Shimoyama T, Okuda M, Kato C, Kobayashi I, Fujioka T. Influence of proton pump inhibitor treatment on
Helicobacter pylori stool antigen test. World J Gastroenterol 2012; 18(1): 44-48 - URL: https://www.wjgnet.com/1007-9327/full/v18/i1/44.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i1.44
The urea breath test (UBT) has shown high sensitivity and specificity in the non-invasive diagnosis of Helicobacter pylori (H. pylori) infection, making the UBT one of the most valuable non-invasive tests for diagnosing H. pylori infection and eradication throughout the world[1]. This test has been recommended in the 2005 revision of the guidelines for H. pylori diagnosis and treatment of the European Helicobacter study group (Maastricht III)[2].
The stool antigen test, using polyclonal and monoclonal antibodies, is another non-invasive method for detecting H. pylori[3], with comparable sensitivity and specificity to the UBT. Maastricht III has also recommended the stool antigen test as a simple, easy and useful method for detecting the presence or eradication of H. pylori[2]. Native catalase was recently identified as an antigen produced by H. pylori[4]. Catalase is characterized by its stability, and the stool antigen test using a monoclonal antibody to catalase is both rapid, taking only about 70 min, and considered more specific than methods using polyclonal antibodies[4]. Although the UBT is non-invasive and the laboratory procedure is recognized as simple and easy, it requires a longer period of time and trained medical staff. Moreover, the UBT is difficult for some patients, including children, handicapped individuals with deteriorated activities of daily life (ADL) and elderly individuals. The stool antigen test is a simpler analytical process, does not require as many medical staff members and can be more readily applied for patients with reduced ADL, children, and the elderly.
Proton pump inhibitors (PPIs) have strong antigastric secretion effects, as well as being bacteriostatic against H. pylori. Since UBT may yield false-negative results in patients being treated with PPIs[5,6], it has been recommended that PPI treatment be suspended for at least 2 wk prior to UBT for H. pylori infection. One of the stool antigen tests available is the Testmate pylori antigen enzyme immunoassay (EIA)[7], which uses native catalase as an antigen. Thus, the precision of this test in diagnosing H. pylori infection should remain stable even during PPI treatment. To determine their comparative accuracy in patients being treated with PPIs, we compared this stool antigen test and the UBT in patients before and after PPI administration.
We assessed 28 patients [16 men and 12 women; mean age, (63.1 ± 5.9) years; range, 25-84 years] who had been referred to the Department of Gastroenterology at Oita University Hospital and diagnosed with peptic ulcer, reflux esophagitis, or other diseases requiring PPI treatment.
The UBT and stool antigen test were performed before and after PPI administration. Patients were treated for > 2 wk with the 3 types of PPI commercially available in Japan: omeprazole (10 mg/d or 20 mg/d); lansoprazole (15 mg/d or 30 mg/d); or rabeprazole (10 mg/d or 20 mg/d).
Breath samples for UBT were collected before and 20 min after each subject took a 100 mg UBIT tablet® (Otsuka Pharmaceutical, Tokyo, Japan). 13CO2/12CO2 ratios were analyzed by INIS (UBiT-IR300; Otsuka Electronics, Tokyo, Japan), with a cut-off point of 2.5‰.
Stool samples were collected from each patient before and after PPI treatment, and stored at -80 °C until assayed. The stool antigen test was performed using the Testmate pylori antigen EIA (Wakamoto Pharmaceutical, Tokyo, Japan; Kyowa Medex, Tokyo, Japan). An aliquot of 100 mg stool was diluted into 0.4 mL of diluted buffer, and 50 μL, together with peroxidase-conjugated anti-catalase monoclonal antibody, were added to a well and incubated for 1 h at 25 °C. Absorbance was measured at wavelengths of 450 nm and 630 nm, with a cut-off value of 0.120.
Statistical comparisons were performed using the χ2 test, the Wilcoxon signed-rank test, and the paired t-test.
Prior to PPI administration, both the UBT and stool antigen tests yielded positive results in 20 of 28 patients and negative results in 5. One patient was positive on the UBT and negative on the stool antigen test, and 2 patients were negative on the UBT and positive on the stool antigen test. Using UBT as the standard, the sensitivity, specificity and agreement of the stool antigen test before PPI treatment were 95.2%, 71.4%, and 89.3%, respectively (Table 1).
| Urea breath test | |||
| Positive | Negative | Total | |
| Stool antigen test | |||
| Positive | 20 | 2 | 22 |
| Negative | 1 | 5 | 6 |
| Total | 21 | 7 | 28 |
Following PPI administration, both the UBT and stool antigen test showed positive results in 16 patients and negative results in 9. Two patients were positive on the UBT and negative on the stool antigen test, and one was showed on the UBT and positive on the stool antigen test. Using UBT as the standard, the sensitivity, specificity and agreement of the stool antigen test after PPI treatment were 88.9%, 90.9%, and 89.3%, respectively (Table 2).
| Urea breath test | |||
| Positive | Negative | Total | |
| Stool antigen test | |||
| Positive | 16 | 1 | 17 |
| Negative | 2 | 9 | 11 |
| Total | 18 | 10 | 28 |
The UBT positivity rates were 75.0% (21/28) before and 64.3% (18/28) after PPI treatment (P = 0.55; Table 3). The stool antigen test positivity rates were 78.6% (22/28) before and 60.7% (17/28 cases) after PPI treatment (P = 0.15). No significant differences in the positivity ratios of the two assays were observed before (P = 0.75) or after (P = 0.58) PPI treatment.
| BeforePPI treatment | AfterPPI treatment | P value | |
| Urea breath test | 75.0% (21/28) | 64.3% (18/28) | 0.55 |
| Stool antigen test | 78.6% (22/28) | 60.7% (17/28) | 0.15 |
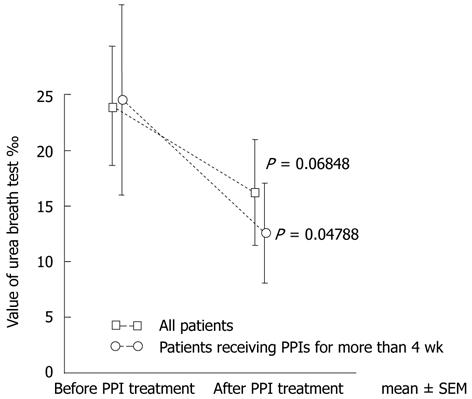
The mean UBT values were 23.98% ± 5.33% before and 16.19% ± 4.75% after PPI treatment (P = 0.068), and were 24.53% ± 8.53% and 12.58% ± 4.49%, respectively (P = 0.048), in the 15 patients treated with PPIs for ≥ 4 wk (Figure 1).
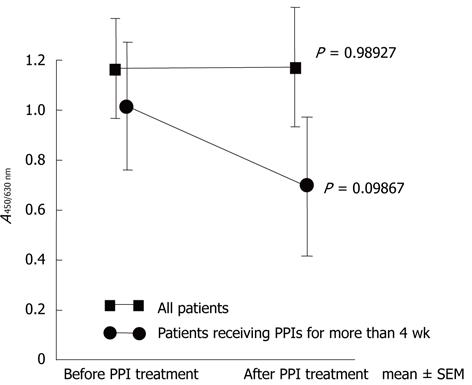
The mean A ratios on the stool antigen test were 1.16 ± 0.20 before and 1.17 ± 0.24 after PPI treatment (P = 0.989), and were 1.02 ± 0.26 and 0.69 ± 0.28, respectively (P = 0.099), in the 15 patients treated with PPI for ≥ 4 wk (Figure 2).
The stool antigen test is a non-invasive test for H. pylori, similar to the UBT. The test is simple and easy to perform, with good sensitivity and specificity[8]. An analysis of 89 reports found that the mean sensitivity, specificity, positive predictive value and negative predictive value were 91%, 93%, 92%, and 87%, respectively, among 10 858 subjects who were not treated with H. pylori eradication therapy[3]. In addition, 8 reports of tests with monoclonal antibody showed significantly better results (P < 0.001), with mean sensitivity, specificity, positive predictive value and negative predictive value of 96%, 97%, 96%, and 97%, respectively. The Maastricht III guidelines have found that the reliability of the stool antigen test is comparable to that of the UBT[2].
Despite its non-invasive nature, and high sensitivity and specificity, the UBT can lead to false-negative results in patients treated with drugs showing bacteriostatic activity against H. pylori, such as PPIs, or inhibiting urease activity[5,6,9-12]. In those studies, the false-negative rates of the UBT in patients treated with omeprazole or lansoprazole for 2 wk or 4 wk were ≥ 50%. We previously observed false-negative UBT results in 1 of 16 patients (6.3%) treated with 30 mg/d lansoprazole for 2 wk[6]. Several studies have also reported that the rate of false-negative results on the stool antigen test also increase in patients treated with PPI[13,14]. A comparison of stool antigen test and UBT results for 9 H. pylori-positive patients receiving PPI for 2 wk found a smaller degree of change on the stool antigen test than on the UBT[15].
The authors found that, before PPI treatment, the stool antigen test showed high sensitivity, but lower specificity (71.4%). This was likely due to the small number of patients showing negative results on the UBT (n = 7). Similarly, although many reports have shown high sensitivity and specificity for stool antigen tests, others have reported lower specificity (54%-78%)[3].
The sensitivity of the stool antigen test fell slightly after PPI administration, but its specificity remained high level (90.9%). The concordance rate of UBT and stool antigen test results was high (89.3%) before and after PPI administration. Using the UBT as standard, the stool antigen test showed good sensitivity and specificity both before and after PPI administration. Although stool antigens have generally shown high sensitivity and specificity, divergent sensitivity and specificity between the UBT and stool antigen test have been reported[16]. However, increasing the cut-off for the stool antigen test reduced the percentage of conflicting results[16]. This discrepancy was attributed to the urease-based UBT not detecting the coccoid form of H. pylori, as well as the low cut-off index for the stool antigen test. The discrepancies we observed, with positive results on the stool antigen test and negative results on the UBT, were likely due to the same mechanism.
There were no significant differences in positive rates on the UBT and stool antigen test before and after PPI therapy, suggesting that the stool antigen test is a useful and reliable diagnostic test for H. pylori, similar to the UBT. The positivity rate for UBT decreased from 75.0% before to 64.3% after PPI administration, and the positivity rate for the stool antigen test decreased from 78.6% to 60.7%. These reductions indicate that the stool antigen test should be performed after limiting PPI administration as much as possible, but that, when PPI treatment cannot be stopped, the stool antigen test shows comparable utility to the UBT. Although both the stool antigen test and UBT results did not change significantly from before to after PPI treatment, the reductions were much less pronounced on the stool antigen test than on the UBT. Moreover, in patients treated for ≥ 4 wk, UBT, but not stool antigen test, results decreased significantly after PPI treatment. These findings indicate that, while the results of both assays were influenced by the bacteriostatic actions of PPIs, the stool antigen test was less influenced than the UBT.
Although several studies have reported increased false-negative results for the stool antigen test during PPI treatment[13,14], we found that stool antigen test results were more stable than UBT results in patients being treated with PPIs.
Despite the benefits of the UBT as a non-invasive test for H. pylori[17] and the slightly lower accuracy of the stool antigen test, the latter has several advantages, including ease, rapidity and lower cost. Others have also reported that the stool antigen test is highly sensitive and specific, with high utility due to the speed and simplicity of testing[18].
The 2005 Maastricht III consensus report recommended that the stool antigen test be used for diagnosis when the UBT is unavailable[2]. The numbers of patients receiving PPI therapy have been increasing around the world, and many patients have difficulty undergoing the UBT, such as elderly patients and patients with reduced ADL. In these patients, the stool antigen test should be considered reliable and useful.
In conclusion, the stool antigen test showed stable results even during PPI treatment as well as sensitivity comparable to the UBT. In patients treated with PPI for ≥ 4 wk, the stool antigen test showed more stable results than the UBT. The stool antigen test is therefore a useful and reliable diagnostic method for H. pylori infection.
Although the urea breath test (UBT) is a reliable, non-invasive diagnostic test for Helicobacter pylori (H. pylori), it has a potential for false-negative results in patients treated with proton pump inhibitor (PPI). The stool antigen test is another useful, easy to perform, non-invasive assay for H. pylori, with high sensitivity and specificity. However, the effects of PPI treatment on stool antigen test results are unclear.
Due to the increase in patients with gastroesophageal reflux disease and those treated with nonsteroidal anti-inflammatory drugs, the numbers treated with PPIs has increased throughout the world. Thus, reliable diagnostic tests for H. pylori are needed for patients treated with PPIs. The study describe the utility of a stool antigen test, using the TestMate pylori enzyme immunoassay, in patients treated with PPIs.
Although many studies have reported results of stool antigen tests in patients treated with PPIs, none has assessed the relationship between measured value of stool antigen test and UBT results. To our knowledge, this study is the first to clarify the stability of the stool antigen test, relative to the UBT, in patients treated with PPIs.
Elderly patients and those with reduced daily activities who are treated with PPIs have increased. The UBT is somewhat difficult to perform in these patients. The authors found that the stool antigen test may be a useful alternative to the UBT in these patients.
The stool antigen test is a diagnostic assay for H. pylori. which identifies H. pylori antigens in feces using antigen-antibody reactions. This non-invasive assay has equal sensitivity and specificity to the UBT, and may be especially useful for children or elderly patients.
The manuscript is well written. The methods are adequate. The results justify the conclusions drawn.
Peer reviewers: Julio H Carri, Professor, Internal Medicine-Gastroenterology, National University of Córdoba, Av. Estrada 160-P 5-Department D, Córdoba 5000, Argentina; Mario M D’Elios, Professor, University of Florence, viale Morgagni 85, Florence 50134, Italy
S- Editor Zhang SJ L- Editor O’Neill M E- Editor Zhang DN
| 1. | Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 2004;20:1001-1017. [PubMed] |
| 2. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] |
| 3. | Gisbert JP, Pajares JM. Stool antigen test for the diagnosis of Helicobacter pylori infection: a systematic review. Helicobacter. 2004;9:347-368. [PubMed] |
| 4. | Suzuki N, Wakasugi M, Nakaya S, Kokubo N, Sato M, Kajiyama H, Takahashi R, Hirata H, Ezure Y, Fukuda Y. Catalase, a specific antigen in the feces of human subjects infected with Helicobacter pylori. Clin Diagn Lab Immunol. 2002;9:784-788. [PubMed] |
| 5. | Laine L, Estrada R, Trujillo M, Knigge K, Fennerty MB. Effect of proton-pump inhibitor therapy on diagnostic testing for Helicobacter pylori. Ann Intern Med. 1998;129:547-550. [PubMed] |
| 6. | Murakami K, Sato R, Okimoto T, Watanabe K, Nasu M, Fujioka T, Kodama M, Kagawa J. Influence of anti-ulcer drugs used in Japan on the result of (13)C-urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol. 2003;38:937-941. [PubMed] |
| 7. | Suzuki N, Wakasugi M, Nakaya S, Okada K, Mochida R, Sato M, Kajiyama H, Takahashi R, Hirata H, Ezure Y. Production and application of new monoclonal antibodies specific for a fecal Helicobacter pylori antigen. Clin Diagn Lab Immunol. 2002;9:75-78. [PubMed] |
| 8. | Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [PubMed] |
| 9. | Chey WD, Woods M, Scheiman JM, Nostrant TT, DelValle J. Lansoprazole and ranitidine affect the accuracy of the 14C-urea breath test by a pH-dependent mechanism. Am J Gastroenterol. 1997;92:446-450. [PubMed] |
| 10. | Connor SJ, Seow F, Ngu MC, Katelaris PH. The effect of dosing with omeprazole on the accuracy of the 13C-urea breath test in Helicobacter pylori-infected subjects. Aliment Pharmacol Ther. 1999;13:1287-1293. [PubMed] |
| 11. | Savarino V, Bisso G, Pivari M, Zentilin P, Bilardi C, Dulbecco P, Mele MR, Tracci D, Vigneri S. Effect of gastric acid suppression on 13C-urea breath test: comparison of ranitidine with omeprazole. Aliment Pharmacol Ther. 2000;14:291-297. [PubMed] |
| 12. | Adachi K, Fujishiro H, Mihara T, Komazawa Y, Kinoshita Y. Influence of lansoprazole, famotidine, roxatidine and rebamipide administration on the urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:168-171. [PubMed] |
| 13. | Manes G, Balzano A, Iaquinto G, Ricci C, Piccirillo MM, Giardullo N, Todisco A, Lioniello M, Vaira D. Accuracy of the stool antigen test in the diagnosis of Helicobacter pylori infection before treatment and in patients on omeprazole therapy. Aliment Pharmacol Ther. 2001;15:73-79. [PubMed] |
| 14. | Bravo LE, Realpe JL, Campo C, Mera R, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroenterol. 1999;94:2380-2383. [PubMed] |
| 15. | Fukuda Y, Tomita T, Kosaka T, Hori K. [Evaluation of diagnostic methods for Helicobacter pylori infection]. Nihon Rinsho. 2004;62:459-463. [PubMed] |
| 16. | Masoero G, Lombardo L, Della Monica P, Vicari S, Crocillà C, Duglio A, Pera A. Discrepancy between Helicobacter pylori stool antigen assay and urea breath test in the detection of Helicobacter pylori infection. Dig Liver Dis. 2000;32:285-290. [PubMed] |
| 17. | Manes G, Zanetti MV, Piccirillo MM, Lombardi G, Balzano A, Pieramico O. Accuracy of a new monoclonal stool antigen test in post-eradication assessment of Helicobacter pylori infection: comparison with the polyclonal stool antigen test and urea breath test. Dig Liver Dis. 2005;37:751-755. [PubMed] |
| 18. | Kato S, Ozawa K, Okuda M, Nakayama Y, Yoshimura N, Konno M, Minoura T, Iinuma K. Multicenter comparison of rapid lateral flow stool antigen immunoassay and stool antigen enzyme immunoassay for the diagnosis of Helicobacter pylori infection in children. Helicobacter. 2004;9:669-673. [PubMed] |