Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5710
Revised: September 3, 2010
Accepted: September 10, 2010
Published online: December 7, 2010
AIM: To examine the accuracy of the aspartate aminotransferase (AST)/Platelet Ratio Index (APRI) and FIB-4, in predicting longitudinal changes in liver histology in hepatitis C virus (HCV) patients.
METHODS: Patients that underwent repeat liver biopsies at least 1 year apart from 1999 to 2007 were identified. Liver fibrosis was staged on needle core biopsies evaluated by a single expert liver pathologist. Only laboratory values within 3 mo of the liver biopsies were used.
RESULTS: Thirty-six patients met the inclusion criteria with 50% stage 1 on initial biopsy, 25% stage 2, and 22% stage 3. Nineteen of 36 (53%) had progression of fibrosis on repeat biopsies, while 16 (44%) showed no change in stage, and one (3%) showed improvement. Patients that showed progression of fibrosis had significantly higher alanine aminotransferase and aspartate aminotransferase levels than the group that did not show progression. A significant correlation was seen between change in stage of fibrosis and change in APRI (r² = 0.39, P = 0.00001) and a change in FIB-4 (r² = 0.31, P = 0.00004). A change in APRI (ΔAPRI) of 0.18 had 80% positive predictive value (PPV) and 67% negative predictive value (NPV) for progression of fibrosis. A change in FIB-4 (ΔFIB-4) of 0.39 had 75% PPV and 75% NPV for predicting progression of fibrosis.
CONCLUSION: ΔAPRI and ΔFIB-4 parallel changes in fibrosis progression, and could be useful tools for clinicians in following patients with active chronic HCV infection.
- Citation: Mummadi RR, Petersen JR, Xiao SY, Snyder N. Role of simple biomarkers in predicting fibrosis progression in HCV infection. World J Gastroenterol 2010; 16(45): 5710-5715
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5710.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5710
Hepatitis C virus (HCV) infection is one of the most common causes of chronic liver disease, and affects approximately 3% of the world’s population[1]. Current treatment decisions in chronic HCV are guided by the histopathological findings on liver biopsy. Although it is still considered the gold standard for assessment of fibrosis in chronic hepatitis C, liver biopsy has limitations. It is an expensive and invasive procedure with complications that range from pain (0.06%-22%) to death (0.0088%-0.3%)[2]. Liver biopsy is also limited by both inter-observer and sampling variability. Although inter-observer variability has been improved by standardized scoring systems for fibrosis and inflammation, discordance rates for the stage of fibrosis of approximately 33% have been reported[3,4]. The frequency of sampling errors is not surprising given the fact that a standard percutaneous liver biopsy specimen has been calculated to sample only 1/50 000 part of the liver[5]. Considering the expense and limitations of liver biopsy, there is great interest in the use of non-invasive markers of liver fibrosis that can replace liver biopsy both as a guide to therapy and as a prognostic indicator. Current studies have shown that non-invasive markers can predict the absence of significant disease or the presence of advanced disease at the time of liver biopsy, but it is not clear that they can predict step-wise progression of fibrosis[6]. It is also not clear if they have prognostic value regarding long-term clinical outcomes. If they can be proven to be useful longitudinally, this would be of great benefit to clinicians and their patients.
Non-invasive markers can be broadly divided into two major groups: radiological and serum-based markers. Transient elastography using ultrasound waves to measure stiffness of liver is an example of a radiological method but is limited in terms of cost, technical complexity and availability. It currently is not approved for clinical use in the United States by the Food and Drug Administration. Serum biomarkers have been proposed as an inexpensive and effective alternative to replace liver biopsy and can be subdivide into two categories: (1) indirect markers that are composed of simple routine biochemical and/or hematological tests; and (2) markers composed of substances that are part of the extracellular matrix (ECM). Although simple markers are inexpensive and available universally, the markers of ECM are expensive and available only in reference or research laboratories. Two indirect marker panels, aspartate aminotransferase (AST)/Platelet Ratio Index (APRI) and FIB-4, are composed of panels that are routinely preformed in patients with liver disease, and therefore, could be a practical and convenient way to follow patients[7,8].
Most studies of biomarkers in fibrosis have compared their diagnostic accuracy in predicting the presence of mild vs significant fibrosis, mild/moderate vs advanced fibrosis, or cirrhosis, using a liver biopsy obtained at the same time as the gold standard[6]. This approach has limitations because liver biopsy is a less than ideal reference standard and it is not clear if these biomarkers are useful in identifying patients in whom fibrosis is likely to progress. It is important to develop a non-invasive tool for predicting progression of fibrosis, because these patients are the ones who might benefit from clinical interventions like antiviral therapy and/or more frequent and intense monitoring.
In this study, we examined APRI and FIB-4 in a group of patients with paired liver biopsies to determine if longitudinal changes in the markers correlated with changes in the histological stage of fibrosis, and also examined whether the initial or follow-up APRI and FIB-4 was useful in predicting those patients who showed an increase in stage of fibrosis on liver biopsy.
Following approval by the Institutional Review Board, we searched a database available in the Department of Pathology for all patients who had undergone at least two liver biopsies for staging fibrosis in mono-infected HCV-positive patients between 1999 and 2007. Patients were selected for inclusion if the liver biopsies were at least 1 year apart. Patients were excluded if they had cirrhosis, were co-infected with hepatitis B virus (HBV) or human immunodeficiency virus, had an organ transplant, daily alcohol intake of > 30 g/d, hepatocellular carcinoma or primary metabolic or autoimmune liver disease. The laboratory values used for the calculations of the APRI and FIB-4 were those that were closest to the liver biopsy date. Our hepatology service has had an ongoing prospective study of hepatic fibrosis markers since 2003, and 20 of the patients were enrolled in that study for one or both of their liver biopsies. Those patients had their blood drawn on the day of the liver biopsy. All other patients had blood work within 3 mo of the biopsy, or they were excluded from the study.
The platelets were measured using the Sysmex SE 9500 (Sysmex, Mundelein, IL, USA) and the AST and the alanine aminotransferase (ALT) were measured using either the Vitros 950 or 5,1 FS (Ortho Clinical diagnostics, Raitan, NJ, USA).
The APRI is a numerical value that is calculated using the following formula: APRI = [AST (U/L)/upper limit of normal (U/L)] × 100/platelets (109/L)[7]. The FIB-4 index is also a numerical value that is calculated using the following formula FIB 4 = Age × AST (U/L)/[platelets (109/L) × ALT1/2 (U/L)][8].
All liver biopsies were read by a single pathologist (S.X.), without prior knowledge of the study status or the value of laboratory parameters being tested. Fibrosis was staged as described below. The fibrosis score is based on a five-point scale: stage 0 = no fibrosis; 1 = either mild pericellular fibrosis in the lobules, or mild portal fibrosis; 2 = periportal fibrosis, or portal fibrosis plus lobular pericellular fibrosis; 3 = septal or bridging fibrosis without evident parenchymal remodeling; and 4 = cirrhosis (with architectural remodeling and nodular formation). The rationales of including lobular pericellular fibrosis in our staging system are: (1) that this type of fibrosis is frequently observed among patients with chronic HCV infection; and (2) that zone 3 (or centrilobular) hepatic stellate cell activation is common in hepatitis C liver biopsies without other co-morbidity[9].
In the initial analysis the median AST, ALT, platelets, APRI and FIB-4 scores were compared between patients who had an increase in at least one stage on liver biopsies (progressors) and those who did not progress (non-progressors) using a non-parametric test (Mann-Whitney U test). Logistic regression was used to calculate the accuracy of the ALT, AST, platelets, APRI and FIB-4 in predicting progression of fibrosis. Receiver operating characteristic (ROC) curves were calculated for the biomarkers at the time of initial and repeat biopsies. Area under the ROC curve (AUROC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Non-parametric tests were performed using Stats Direct statistical software (Sale, Cheshire, UK) and ROC curves were constructed using MedCalc statistical software (Marialterke, Belgium).
Assuming that the AUROC for APRI and FIB-4 would be 0.8 if the null hypothesis is rejected and 0.5 if the null hypothesis is valid, a sample size of 29 is needed to detect the difference at an α value of 0.05 and β value of 0.8.
A total of 36 patients met the inclusion criteria. The baseline characteristics of the subjects are described in Table 1. The median age at the time of initial biopsies was 47 years (range: 25-68 years). Seventy-five percent (27/36) of patients were male, with 50% (18/36) Caucasians, 31% (11/36) African Americans and 19% (7/36) Hispanics. The majority (50%) of the patients were stage 1 on biopsy followed by stage 2 (25%) and stage 3 (22%). One patient had stage 0 fibrosis on biopsy, and there were no patients with cirrhosis because they were excluded from the study. The median duration between biopsies was 4 years (range: 2-9 years).
| n = 36 | |
| No. of males, n (%) | 27 (75) |
| Age (yr, range) | 47 (25-68) |
| Race , n (%) | |
| Caucasian | 18 (50) |
| African American | 11 (31) |
| Hispanic | 7 (19) |
| Stage at time of initial biopsy, n (%) | |
| Stage 0 | 1 (3) |
| Stage 1 | 18 (50) |
| Stage 2 | 9 (25) |
| Stage 3 | 8 (22) |
| ALT (IU/mL, range) | 73 (14-322) |
| AST (IU/mL, range) | 49 (22-266) |
| Platelets (× 109/L, range) | 235 (185-362) |
| Median duration between biopsies (yr, range) | 4 (2-9) |
Out of a total of 36 patients, 19 (53%) had progression of fibrosis on repeat biopsies, 16 patients (44%) showed no change in stage, and one (3%) showed improvement in fibrosis on repeat biopsy. Among the patient factors examined (Table 2), the group that showed progression had significantly higher ALT and AST at baseline when compared to the group that did not show progression (P = 0.003 and 0.0001, respectively). There was no statistically significant difference between baseline APRI, FIB-4 index, stage at initial biopsy, age, or duration between biopsies between progressors and non-progressors.
| Progressors | Non-progressors | P value | |
| No. | 19 (14 with a 1-stage change; 5 with a 2-stage change) | 17 | 0.999 |
| Age (yr) | 50 | 52 | 0.621 |
| Sex | Male 13 | Male 14 | > 0.050 (NS) |
| Female 6 | Female 3 | ||
| APRI | 0.66 | 0.56 | 0.173 |
| FIB-4 | 1.307 | 1.347 | 0.268 |
| ALT (IU/mL) | 92 | 47 | 0.003 |
| AST (IU/mL) | 100 | 38 | 0.001 |
| Platelets (× 109/L) | 235 | 243 | 0.956 |
| Stage | 2 | 1 | 0.477 |
| Duration between biopsies (yr) | 4 | 4 | 0.200 |
Progressors vs non-progressors: Differences in parameters were explored between the two groups at the time of repeat biopsies. ALT, AST, APRI and FIB-4 were significantly higher in patients who showed progression of fibrosis (Table 3). The APRI and FIB-4 at the time of the second biopsy had excellent predictive value for progression in fibrosis with an APRI ≥ 0.69 having a 79% PPV and a 78% NPV for progression of at least one stage of fibrosis. A FIB-4 ≥ 1.65 had an 81% PPV and 70% NPV for prediction of fibrosis by at least one stage. A one-stage change in fibrosis on repeat biopsies might reflect sampling variability, therefore, we examined the predictive value of the APRI and FIB-4 for a change of two stages of fibrosis. We found that the APRI and FIB-4 had excellent predictive values for a two-stage progression of fibrosis with an APRI ≥ 1.94 and FIB-4 ≥ 3.01 having a 100% PPV and NPV and 62% PPV and 100% NPV, respectively. In addition the APRI had an AUROC of 1.0 [95% confidence interval (CI): 0.85-1.00] and FIB-4 had an AUROC of 0.911 (95% CI: 0.717-0.986, P = 0.0001) for those who had a two-stage change in fibrosis. The number of patients who progressed by two stages was low (n = 5), and caution should be exercised in interpreting this particular subset of the results.
| Progressors | Non-progressors | P value | |
| APRI | 1.293 | 0.413 | 0.0006 |
| FIB-4 | 2.816 | 1.36 | 0.0038 |
| ALT (IU/mL) | 92 | 47 | 0.0030 |
| AST (IU/mL) | 100 | 38 | 0.0001 |
| Platelets (× 109/L) | 184 | 221 | 0.1160 |
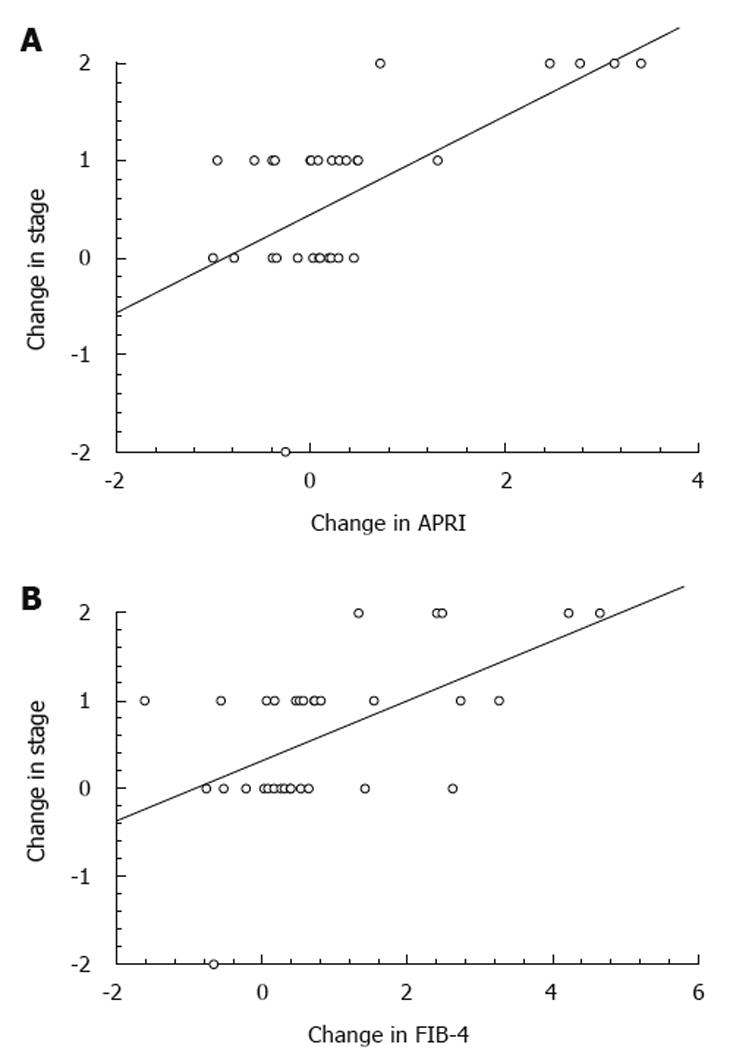
Change in APRI and FIB-4 and progression vs non-progression: Changes in APRI and FIB-4 were correlated with change in stage of fibrosis. There was a significant correlation between change in stage and change in APRI (r² = 0.39, P = 0.00001) (Figure 1A) with the regression equation for a change in stage = 0.50 × (ΔAPRI) + 0.44. AUROCs were constructed for predictive value of change in APRI for change in stage with a ΔAPRI of 0.18 having an 80% PPV and 67% NPV in predicting progression of fibrosis. Similarly, the correlation between change in stage and ΔFIB-4 was also significant (r² = 0.31, P = 0.00004) (Figure 1B). The regression equation for a change in stage = 0.34 × (ΔFIB-4) + 0.32. An analysis of ROC curve revealed that a ΔFIB-4 of 0.39 had 75% PPV and NPV for predicting progression of fibrosis.
Accuracy of individual tests: AST proved to be the most useful of the tests. There was a significant correlation between change in stage of fibrosis and change in AST (r2 = 0.33, P =0.002). There was a less but still significant correlation of ALT between change in fibrosis and change in ALT ( r2 = 0.21, P =0.0048). Changes in the platelets alone did not correlate well with changes in fibrosis (r2 = 0.07).
Liver biopsy is the gold standard for estimating severity of fibrosis in patients with liver disease and is used as a guide for therapy of HCV infection. However, because antiviral therapy with pegylated interferon and ribavirin carries significant toxicity, and fibrosis progresses in only 20%-30% of patients with chronic HCV infection[10], therapy is ideally offered to patients whose risk of progressive liver damage is high. Current strategy involves using the stage of fibrosis on initial liver biopsy to determine the risk of progressive liver damage. However, the stage at the time of the biopsy might not predict the risk of progression, especially in a younger patient and/or someone with a shorter duration of infection.
Apart from the complications associated with the invasive nature of liver biopsy, issues like sampling variability, inter-observer variability, and expense limit its utility and diagnostic accuracy[9,11]. Factors that determine the varying rates of fibrosis progression are poorly understood, partially because of the logistical difficulties for studies in performing serial liver biopsies. Non-invasive biomarkers that reflect hepatic fibrosis have been proposed to overcome these limitations with the ideal biomarker of hepatic fibrosis being inexpensive, easily available, and accurately reflecting changes in fibrosis. The APRI and FIB-4 are two biomarker panels that incorporate readily available and routinely performed tests for all patients with liver disease. Both have been confirmed to be accurate in predicting fibrosis stage on liver biopsies[1,7,8,12-23]. However, few studies have examined the longitudinal behavior of fibrosis biomarkers. Patel et al[24] have evaluated a single direct hepatic fibrosis marker, hyaluronic acid (HA), in patients undergoing liver biopsies before, and 6 mo following antiviral therapy for chronic HCV. Although HA has some association with the stage of fibrosis, it does not predict histological changes over the treatment period. Our study addressed the longitudinal behavior of these markers in individuals who underwent serial biopsies, and examined the role of these biomarkers in predicting progression.
There is accumulating evidence that hepatic fibrosis markers can be used for prognosis. Following 6 mo of interferon-based therapy, an APRI > 1.5 has been shown to be associated with significant short-term mortality and risk of hepatocellular carcinoma in patients with chronic HCV[21]. A commercially available test, FibroTest, has been found at baseline to predict outcomes in chronic HCV[25]. In addition, two other commercially available tests, Fibrometer and Hepascore, have been shown to be as good as liver biopsy in the prediction of survival in alcoholic liver disease[26]. Recently, the European Liver Fibrosis Test has been shown to be superior to several other parameters in the prediction of outcomes of primary biliary cirrhosis[27].
One finding of our study that is potentially useful for clinicians is that serial changes in APRI and FIB-4, which are easily calculated using routine laboratory tests, correlated well with changes in fibrosis staging. There was a significant correlation between change in stage and change in APRI and FIB-4 of 0.627 (r² = 0.39, P = 0.00001) and 0.56 (r² = 0.31, P = 0.00004), respectively. A ΔAPRI of 0.18 had 80% PPV and 67% NPV in predicting progression of fibrosis, whereas a ΔFIB-4 of 0.39 had 75% PPV and NPV for predicting progression of fibrosis. These results are significant as they suggest that longitudinal changes of APRI and FIB-4 markers can be useful in predicting progression of fibrosis, which could lead to changes in clinical management.
The baseline characteristics of the study population did not differ between progressors and non-progressors, except for aminotransferases, which were significantly higher in the group that later showed progression of fibrosis, which has been shown previously[28]. This was independent of the initial stage of fibrosis and did not differ significantly between groups. It seems likely that, even with the same stage of fibrosis, higher enzyme levels are a marker for subsequent progression. The effect of timing between the biopsies is unlikely to have an impact on the conclusions because there was no significant difference between duration of biopsies between the progressor and non-progressor groups. As expected, the APRI and FIB-4 were significantly higher at the time of the second liver biopsy in the patients with progression of fibrosis, which reflected the accuracy of these tests at a single point in time. Therefore, not unexpectedly, the APRI and FIB-4 were predictive of progression at the time of the second biopsy. Although the number of patients was small, both tests were able to predict a two-stage change in fibrosis at the second biopsy.
This study had several limitations, mainly due to the largely retrospective nature of the study. Previously, we have found that the APRI was less accurate retrospectively than prospectively[16]. This might have been because the retrospective patient group was more heterogenous, as well as the fact that laboratory values were not always performed close to the time of the biopsy. In addition, concurrent illnesses that could influence aminotransferases and platelets could not be easily determined in some of the patients. Another significant limitation was the relatively small sample size. A larger and well-designed prospective trial that could confirm the role of biomarkers, including those from the ECM, in predicting fibrosis progression would be very useful.
In summary, the APRI and FIB-4 at initial liver biopsy were not useful in predicting patients whose fibrosis would be progressive. However, as previously reported[28], patients with higher aminotransferases appear to have an increased risk of progression of fibrosis. Importantly, the longitudinal use of the APRI and FIB-4 is accurate in predicting progression of fibrosis, and could be useful to clinicians that follow patients with chronic HCV. Based on our data, a ΔAPRI of 0.18 or a ΔFIB-4 of 0.39 in a patient with chronic HCV suggests progression in fibrosis of at least one stage, and could be used to trigger a reconsideration of antiviral therapy and/or liver biopsy. Larger, prospective studies are needed to validate the findings of our study.
The complications of chronic liver disease are largely related to the development of fibrosis. Medical treatments are aimed at prevention of progression of cirrhosis. A liver biopsy has been the usual way to estimate fibrosis. However, it is invasive and expensive, and can be inaccurate because of variability in the degree of fibrosis in parts of the liver that can be sampled. The use of certain serum biomarkers to estimate fibrosis has been found to useful, especially in chronic hepatitis C. These biomarkers can be composed of a combination of simple tests that are used to assess liver function, as well as more complex indices that measure substances in the blood that can originate in the extracellular matrix of the liver itself.
Although several simple and complex biomarkers have been found to be accurate at differentiating mild from significant fibrosis, there has been little evaluation of them in a longitudinal manner. In other words, it is not clear whether changes over time in the value of the markers reflect actual changes in the degree of fibrosis. If these markers can accurately predict changes in fibrosis, then this would be useful to clinicians. Not only could this lead to fewer liver biopsies, but it could lead to changes or initiation of treatment. This study evaluated two simple biomarkers, the aspartate aminotransferase (AST)/Platelet Ratio Index (APRI) and the FIB4, longitudinally in a group of patients with chronic hepatitis who had two liver biopsies that were at least 1 year apart. It found that both biomarkers could accurately predict changes in the stage of fibrosis.
The APRI utilizes AST and platelets, which are simple tests obtained in all liver patients. It was originally described by Wai et al in 2003 (Hepatology 38: 518-526). Its usefulness has been confirmed in multiple studies including that of Snyder et al (J Clin Gastroenterol 2006; 40: 535-542). The FIB4 utilizes AST, platelets, alanine aminotransferase (ALT), and age. It was originally described by Sterling et al in 2006 (Hepatology 43: 1317-1325). A high APRI score at 6 mo following treatment has been shown to correlate with poor survival, as well as the development of hepatocellular carcinoma.
This article shows that physicians can accurately follow patients with chronic hepatic C that have failed previous antiviral therapy or that have not been treated. Treatment decisions can be based on changes in these indices. These findings should be confirmed in larger studies from other institutions. Also, further research should be done with the longitudinal use of the more complex biomarkers, as well as the longitudinal use of simple and complex biomarkers in diseases other than chronic hepatitis C.
Liver fibrosis refers to the amount of scar tissue in the liver. This impairs blood flow and eventually can lead to cirrhosis. AST is an enzyme that is in liver cells and is released when there is active liver damage. ALT is an enzyme specific to liver cells, which is released when there is liver damage. The platelets are a component of the blood that helps with clotting. Platelets decrease with advancing liver disease and fibrosis because some of them are sequestered in the spleen, which enlarges with liver disease, and also a hormone called thrombopoeitin that stimulates platelets production is released in smaller quantities with advancing disease. Biomarkers are substances that can be objectively measured that are used as an indicator of a biological state. They are being increasingly used in medicine as non-invasive ways to screen for and evaluate various diseases including cancer.
The authors show that evolutionary changes in simple scores (APRI and FIB-4) based on routine biochemical parameters can be of value in predicting the evolution of liver fibrosis in chronic hepatitis C. The data provided in this study have important clinical implications that pertain to the management of patients with chronic hepatitis C.
Peer reviewer: Jesús Prieto, Professor, Clinica Universitaria, University of Navarra, Avda, Pio XII, 36, Pamplona 31080, Spain
S- Editor Sun H L- Editor Kerr C E- Editor Lin YP
| 1. | Gangadharan B, Antrobus R, Dwek RA, Zitzmann N. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin Chem. 2007;53:1792-1799. [Cited in This Article: ] |
| 2. | Olthoff KM, Reddy KR. First, do no harm: The question of liver biopsy in living liver donors. Liver Transpl. 2008;14:420-422. [Cited in This Article: ] |
| 3. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [Cited in This Article: ] |
| 4. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [Cited in This Article: ] |
| 5. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [Cited in This Article: ] |
| 6. | Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106. [Cited in This Article: ] |
| 7. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [Cited in This Article: ] |
| 8. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [Cited in This Article: ] |
| 9. | Lau DT, Luxon BA, Xiao SY, Beard MR, Lemon SM. Intrahepatic gene expression profiles and alpha-smooth muscle actin patterns in hepatitis C virus induced fibrosis. Hepatology. 2005;42:273-281. [Cited in This Article: ] |
| 10. | Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24 Suppl 2:3-8. [Cited in This Article: ] |
| 11. | Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38:427-432. [Cited in This Article: ] |
| 12. | Adler M, Gulbis B, Moreno C, Evrard S, Verset G, Golstein P, Frotscher B, Nagy N, Thiry P. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology. 2008;47:762-763; author reply 763. [Cited in This Article: ] |
| 13. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [Cited in This Article: ] |
| 14. | Le Calvez S, Thabut D, Messous D, Munteanu M, Ratziu V, Imbert-Bismut F, Poynard T. The predictive value of Fibrotest vs. APRI for the diagnosis of fibrosis in chronic hepatitis C. Hepatology. 2004;39:862-863; author reply 863. [Cited in This Article: ] |
| 15. | Lin CS, Chang CS, Yang SS, Yeh HZ, Lin CW. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern Med. 2008;47:569-575. [Cited in This Article: ] |
| 16. | Ramos Paesa C, Marcilla F, López G, Hueso E, Pascual A, Aguirre JM. [Valuation of APRI and Forns models for non-invasive diagnosis of fibrosis in patients with hepatitis C in coinfected and non-coinfected with HIV]. An Med Interna. 2007;24:369-374. [Cited in This Article: ] |
| 17. | Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912-921. [Cited in This Article: ] |
| 18. | Snyder N, Gajula L, Xiao SY, Grady J, Luxon B, Lau DT, Soloway R, Petersen J. APRI: an easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J Clin Gastroenterol. 2006;40:535-542. [Cited in This Article: ] |
| 19. | Snyder N, Nguyen A, Gajula L, Soloway R, Xiao SY, Lau DT, Petersen J. The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin Chim Acta. 2007;381:119-123. [Cited in This Article: ] |
| 20. | Trang T, Petersen JR, Snyder N. Non-invasive markers of hepatic fibrosis in patients co-infected with HCV and HIV: comparison of the APRI and FIB-4 index. Clin Chim Acta. 2008;397:51-54. [Cited in This Article: ] |
| 21. | Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, Lee LP, Lin ZY, Hsieh MY, Wang LY. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086-1097. [Cited in This Article: ] |
| 22. | Shaheen AA, Myers RP. Systematic review and meta-analysis of the diagnostic accuracy of fibrosis marker panels in patients with HIV/hepatitis C coinfection. HIV Clin Trials. 2008;9:43-51. [Cited in This Article: ] |
| 23. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [Cited in This Article: ] |
| 24. | Patel K, Lajoie A, Heaton S, Pianko S, Behling CA, Bylund D, Pockros PJ, Blatt LM, Conrad A, McHutchison JG. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol. 2003;18:253-257. [Cited in This Article: ] |
| 25. | Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896. [Cited in This Article: ] |
| 26. | Naveau S, Gaudé G, Asnacios A, Agostini H, Abella A, Barri-Ova N, Dauvois B, Prévot S, Ngo Y, Munteanu M. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology. 2009;49:97-105. [Cited in This Article: ] |
| 27. | Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, Markin RS, Rubin R, Wheeler D, Contos M, West AB. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48:1549-1557. [Cited in This Article: ] |
| 28. | Perumalswami P, Kleiner DE, Lutchman G, Heller T, Borg B, Park Y, Liang TJ, Hoofnagle JH, Ghany MG. Steatosis and progression of fibrosis in untreated patients with chronic hepatitis C infection. Hepatology. 2006;43:780-787. [Cited in This Article: ] |