Copyright
©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 28, 2010; 16(44): 5582-5587
Published online Nov 28, 2010. doi: 10.3748/wjg.v16.i44.5582
Published online Nov 28, 2010. doi: 10.3748/wjg.v16.i44.5582
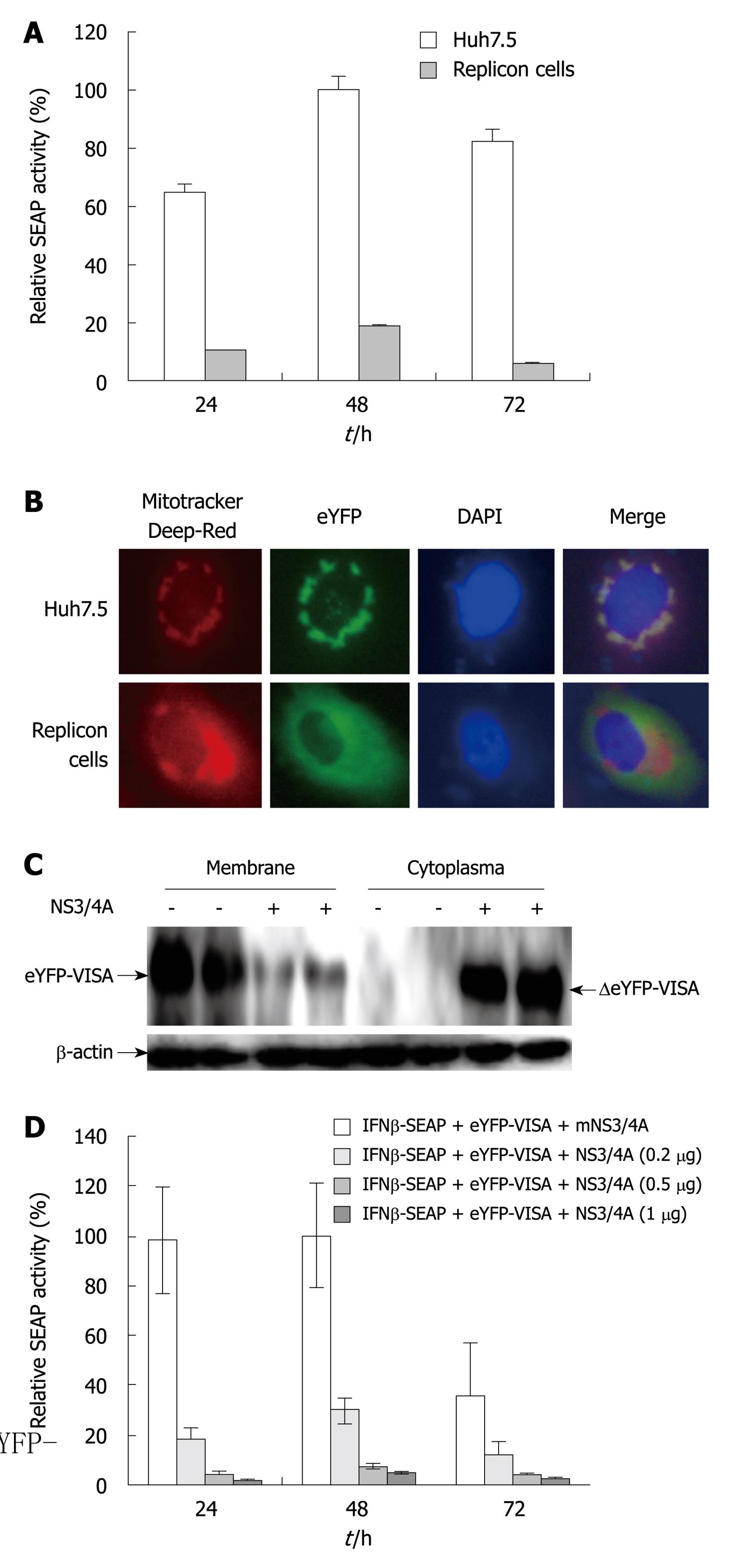
Figure 2 Hepatitis C virus NS3/4A protease activity impairs the enhanced yellow fluorescent protein-mitochondrial antiviral signaling protein/interferon-β-secreted placental alkaline phosphatase signaling pathway.
A: Validation of the reporter assay system in Huh7.5 cells that contain full-length hepatitis C virus (HCV) replicons (P < 0.05). Huh7.5 and replicon cells were co-transfected with enhanced yellow fluorescent protein (eYFP)-mitochondrial antiviral signaling protein (MAVS) and interferon (IFN)-β-secreted placental alkaline phosphatase (SEAP). pRL-TK was co-transfected to normalize transfection efficiency. SEAP activity was examined at 24, 48 and 72 h after transfection. Bars indicate SD (n = 3); B: Localization of eYFP-MAVS. Subcellular localization of eYFP-MAVS was assessed by fluorescence microscopy 48 h post-transfection in Huh7.5 and replicon cells; C: Western blotting analysis of eYFP-MAVS cleaved by NS3/4A protease. Lysates of Huh7.5 and replicon cells treated as above were harvested at 48 h post-transfection and analyzed by Western blotting. Arrows indicate the positions of eYFP-MAVS and ΔeYFP-MAVS, respectively; D: Huh7.5 cells were co-transfected with eYFP-MAVS, IFN-β-SEAP and increasing amounts of expression plasmid pNS3/4A that encoded HCV NS3/4A protease (0, 0.2, 0.5 and 1 μg). pRL-TK was co-transfected to normalize transfection efficiency. SEAP activity in cell culture was measured at 24, 48 and 72 h post-transfection. Bars indicate SD (n = 3).
- Citation: Fu QX, Wang LC, Jia SZ, Gao B, Zhou Y, Du J, Wang YL, Wang XH, Peng JC, Zhan LS. Screening compounds against HCV based on MAVS/IFN-β pathway in a replicon model. World J Gastroenterol 2010; 16(44): 5582-5587
- URL: https://www.wjgnet.com/1007-9327/full/v16/i44/5582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i44.5582