Published online Dec 14, 2009. doi: 10.3748/wjg.15.5879
Revised: August 18, 2009
Accepted: August 25, 2009
Published online: December 14, 2009
The occurrence of concomitant aortic aneurysm and colorectal cancer is a rare medical entity, and controversy surrounds its optimal treatment. We report a case of rectal cancer and concomitant aneurysm from the ascending aorta to the common iliac artery. As with DeBakey type I aortic dissecting aneurysm, our patient was treated by rectal cancer resection, with preservation of the anus (Dixon operation) under controlled hypotension. Blood pressure was maintained at 80-90/50-60 mmHg and the pulse at 70-90 beats/min. The pathological examination of the surgical specimen showed a poorly differentiated T3N0 tumor. The patient had an uneventful recovery without aneurysm rupture, and was discharged from hospital on postoperative day 15 after 3 d adjuvant chemotherapy with oxaliplatin combined with calcium folinate and fluorouracil. The patient was given six courses of adjuvant chemotherapy in 6 mo, without recurrence or metastasis, and the aneurysm was still stable after 2 years follow-up.
- Citation: Lu PH, Tao GQ, Shen W, Cai B, Jiang ZY, Sun J. Surgery for rare aneurysm associated with colorectal cancer. World J Gastroenterol 2009; 15(46): 5879-5881
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5879.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5879
The surgical treatment of coexistent aortic aneurysm and colorectal cancer (CRC) needs special consideration. The controversy is mainly whether to treat both diseases or treat either of them alone. If we choose to treat both, should we treat them simultaneously or as staged procedures? For a two-stage process, there is a significant risk of aortic aneurysm rupture, and a single-stage procedure has the disadvantages of technical difficulty and graft infection[1-3]. Lin et al[4] have proposed that the treatment priority should be given to life-threatening lesions. Such cases include the presence of an abdominal aorta aneurysm with a dangerously large diameter and synchronous obstructing or perforating CRC. Veraldi et al[5] have indicated that a one-stage procedure reduces the length of stay, avoids further surgical and anesthetic trauma, and does not increase graft infection.
We report a case of rectal cancer and concomitant aneurysm from the descending aorta to the common iliac artery, which is similar to a DeBakey type I aortic dissecting aneurysm, with a maximum diameter of 4.06 cm in the abdominal aortic segment and 6.51 cm in thoracic aortic segment. The patient was treated with the Dixon operation under conditions of controlled hypotension, without aneurysm rupture. At 2 years follow-up, the patient remained free of CRC and his aneurysm was stable.
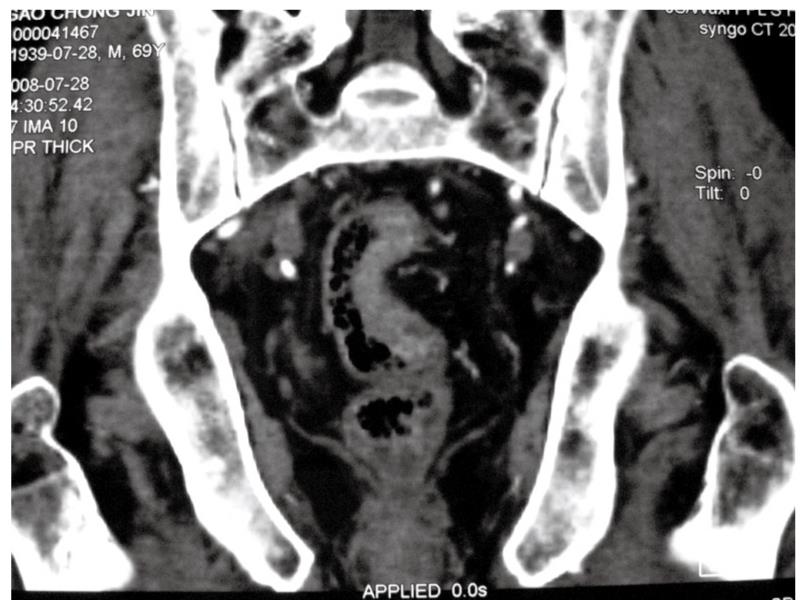
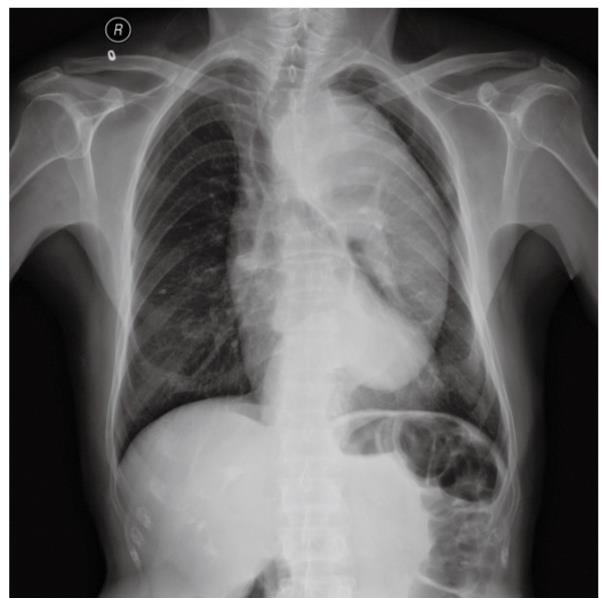
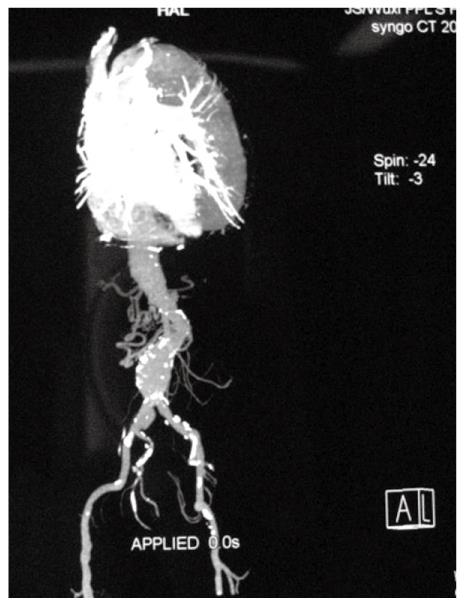
A 69-year-old man underwent anal examination after complaining of a change in bowel habits and rectal bleeding 2 years ago. Anal examination revealed a cauliflower-like lesion located 7 cm from the edge of the anus, which involved more than half of the rectal circumference. Abdominal computed tomography (CT) revealed the presence of a rectal tumor, which almost obstructed the bowel lumen (Figure 1). Medical history included smoking, diabetes, hypertension and thoracoabdominal aortic aneurysm for 6 years. After admission, the patient underwent chest radiography, which confirmed concomitant aneurysm and tracheal compression (Figure 2). Advanced CT angiography revealed large ectasia of the ascending aorta, aortic arch and descending aorta, with a maximum diameter of 6.51 cm and mural thrombosis. In addition, the aneurysm extended to the abdominal aorta, internal-organ arteries and the right common iliac artery, with a length of 60 cm and a diameter of 4.06 cm in the abdominal aortic aneurysm (Figure 3). Elevation of carcinoembryonic antigen and blood urea nitrogen to 11.79 ng/mL and 13.60 mmol/L, respectively, allowed diagnosis of digestive tract tumor and renal inadequacy. A complete preoperative metastatic work-up was negative.
Complicating aneurysm increases the difficulty of managing advanced malignant disease. We treated this patient with the Dixon operation under conditions of controlled hypotension, with minimal blood pressure fluctuation, and renal function protection and blood sugar regulation. Intraoperative blood pressure was maintained at 80-90/50-60 mmHg and the pulse at 70-90 beats/min. The surgery was completed in 1.5 h. Postoperative blood pressure was < 100/60 mmHg and was maintained between 100/60 mmHg and 110/70 mmHg by glyceryl trinitrate administered by mini pump. The pathological examination of the surgical specimen showed a poorly differentiated T3N0 tumor.
The patient had an uneventful recovery, and he was discharged from hospital on postoperative day 15 after 3 d adjuvant chemotherapy with oxaliplatin combined with calcium folinate and fluorouracil. The patient was given six courses of adjuvant chemotherapy without tumor recurrence or metastasis and the aneurysm was still stable after 2 years follow-up.
The concomitant occurrence of aortic aneurysm and CRC appears to be increasing, from 0.5% to 2%[2], as a result of the increase in the two individual diseases, which is caused by increased life expectancy and improved imaging capability in revealing additional asymptomatic disease[5]. Published studies are either small case series or limited retrospective reports[3,6], therefore, the ideal treatment strategy represents a therapeutic dilemma[2]. Initial aortic aneurysm repair followed by CRC resection exposes patients to the risk of tumor progression before resection, although no study has shown the oncological implications of such a delay. Resection of CRC followed by staged aneurysmorrhaphy carries a significant risk of aortic aneurysm rupture in the perioperative period, particularly when the aortic aneurysm is > 5 cm in diameter[2]. Synchronous treatment of both lesions has been described but has the disadvantages of technical difficulties and graft infection, especially when the patient is in poor general condition.
Veraldi et al[5] have found, from Medline 1987-2005, a total of 229 cases of associated aortic aneurysm and CRC. Four emergency operations have been reported, one for rupture of the aortic aneurysm, two for unstable aneurysm, and one for diastatic rupture of the cecum by the stenosing neoplasm in the sigmoid colon. Including these four, there were 25 patients in all with only one disease treated. In 24 of these, intervention was carried out for CRC because of the advanced stage of the disease or poor general condition of the patient. In the remaining case, because of postoperative death, only the aneurysm was treated. From January 1988 to May 2006, 14 patients with both diseases were observed and treated in the University of Verona[5]. In one of these patients, only CRC was treated because of poor cardiac conditions, and the patient died after 2 mo from myocardial infarction.
Patients in poor general condition, who are treated for both diseases simultaneously or in stages face the risk of surgical fatality. Our patient had a rare true aneurysm similar to a dissecting aneurysm[7]. Usually, dissecting aneurysm is caused by an intimal tear or interstitial hemorrhage and requires emergency surgery, and has a low survival rate. He had been protected by blood pressure control since his aneurysm was diagnosed 8 years ago. CT revealed that the aneurysm was in a steady state, and the diameter and distribution of the aneurysm showed no obvious changes in these 8 years. The aneurysm in our patient began from the ascending aorta and extended to the common iliac artery, which is a very rare occurrence, and the prognosis was beyond our expectation. This kind of aneurysm usually requires Bentall’s procedure and the elephant trunk technique for artificial blood vessel replacement. Bentall’s procedure is a cardiac surgery technique that involves composite graft replacement of the aortic valve, aortic root and ascending aorta, with reimplantation of the coronary arteries into the graft. For the elephant trunk technique, excess tubular graft material is inserted during ascending aortic and arch repair, to facilitate the subsequent treatment of distal aortic aneurysms. These operations are extremely hazardous.
Davies et al[8] have pointed out that treatment decisions should involve a balance between the risk of complications caused by the dilated aorta and those from the operation itself. The most devastating complication of an aneurysm is dissection, which leads to arterial occlusion and rupture and is almost invariably fatal. However, the risk of operation is also unmanageable. The risk of spinal cord injury, particularly in operations on the descending aorta[9], is also significant. As a result of surgical risk and expense, our patient elected for conservative treatment for his aneurysm 8 years ago. When we were faced with the rare aortic aneurysm and CRC, we chose only to resect the CRC as a result of the great risks of the operation because of the patient’s age, mural thrombosis, renal inadequacy, diabetes and hypertension. The postoperative recovery was satisfactory, with stable aneurysm and no cancer recurrence during follow-up.
Most surgeons agree that treatment priority should be focused on symptomatic or more life-threatening lesions in CRC and aneurysm. The largest retrospective study to date was reported by Lin et al[4] in 2007. In that study, 108 patients with synchronous aortic aneurysm and CRC were identified, and 92 were treated for both lesions. Thirty-five patients had CRC removed first, following two patients with aneurysm rupture while 2/35 people with aneurysm rupture had CRC removed first. Twenty-three patients with endovascular aortic repair were associated with shorter recovery and lower postoperative mortality rate. This modality offered potential treatment benefits in patients with suitable anatomy who have concomitant CRC. However, the treatment should be offered with caution because of the risk of sigmoid ischemia caused by inferior mesenteric artery occlusion. Sometimes the single operation decision may be a better one. In the study of Lin et al[4], 16 patients were treated for only one disease, because of advanced CRC, hepatic metastasis or ruptured aneurysm, and these patients had a good prognosis. The most important thing is that treatment decisions should involve striking a balance between the risk of the disease itself and the complications of the operation. However, likely aneurysm rupture presents a great challenge, and urgent aneurysm repair is required to preserve the patient’s life.
In summary, in patients with aortic aneurysm and CRC, priority should be given to the more life-threatening lesion. If patients have heart disease or are in poor general condition, attempting to treat both CRC and aneurysm carries a great risk, and it may be possible only to treat one of the conditions.
Peer reviewer: Frank I Tovey, OBE, ChM, FRCS, Honorary Research Felllow, Department of Surgery, University College London, London, United Kingdom
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Rivolta N, Piffaretti G, Tozzi M, Lomazzi C, Riva F, Alunno A, Boni L, Castelli P. Management of simultaneous abdominal aortic aneurysm and colorectal cancer: the rationale of mini-invasive approach. Surg Oncol. 2007;16 Suppl 1:S165-S167. |
| 2. | Baxter NN, Noel AA, Cherry K, Wolff BG. Management of patients with colorectal cancer and concomitant abdominal aortic aneurysm. Dis Colon Rectum. 2002;45:165-170. |
| 3. | Kiskinis D, Spanos C, Melas N, Efthimiopoulos G, Saratzis N, Lazaridis I, Gkinis G. Priority of resection in concomitant abdominal aortic aneurysm (AAA) and colorectal cancer (CRC): review of the literature and experience of our clinic. Tech Coloproctol. 2004;8 Suppl 1:s19-s21. |
| 4. | Lin PH, Barshes NR, Albo D, Kougias P, Berger DH, Huynh TT, LeMaire SA, Dardik A, Lee WA, Coselli JS. Concomitant colorectal cancer and abdominal aortic aneurysm: evolution of treatment paradigm in the endovascular era. J Am Coll Surg. 2008;206:1065-1073; discussion 1074-1075. |
| 5. | Veraldi GF, Minicozzi AM, Leopardi F, Ciprian V, Genco B, Pacca R. Treatment of abdominal aortic aneurysm associated with colorectal cancer: presentation of 14 cases and literature review. Int J Colorectal Dis. 2008;23:425-430. |
| 6. | Illuminati G, Calio' FG, D'Urso A, Lorusso R, Ceccanei G, Vietri F. Simultaneous repair of abdominal aortic aneurysm and resection of unexpected, associated abdominal malignancies. J Surg Oncol. 2004;88:234-239. |
| 7. | Sayer D, Bratby M, Brooks M, Loftus I, Morgan R, Thompson M. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg. 2008;36:522-529. |
| 8. | Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17-27; discussion 27-28. |
| 9. | Griepp RB, Ergin MA, Galla JD, Lansman S, Khan N, Quintana C, McCollough J, Bodian C. Looking for the artery of Adamkiewicz: a quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg. 1996;112:1202-1213; discussion 1213-1215. |