Published online Sep 28, 2009. doi: 10.3748/wjg.15.4571
Revised: August 3, 2009
Accepted: August 10, 2009
Published online: September 28, 2009
AIM: To investigate the usefulness of direct hemoperfusion with a polymyxin B-immobilized fiber column (DHP-PMX therapy) for warm hepatic ischemia-reperfusion (I/R) injury after total hepatic vascular exclusion (THVE) using a porcine model.
METHODS: Eleven Mexican hairless pigs weighing 22-38 kg were subjected to THVE for 120 min and then observed for 360 min. The animals were divided into two groups randomly: the DHP-PMX group (n = 5) underwent DHP-PMX at a flow rate of 80 mL/min for 120 min (beginning 10 min before reperfusion), while the control group did not (n = 6). The rate pressure product (RPP): heart rate × end-systolic arterial blood pressure, hepatic tissue blood flow (HTBF), portal vein blood flow (PVBF), and serum aspartate aminotransferase (AST) levels were compared between the two groups.
RESULTS: RPP and HTBF were significantly (P < 0.05) higher in the DHP-PMX group than in the control group 240 and 360 min after reperfusion. PVBF in the DHP-PMX group was maintained at about 70% of the flow before ischemia and differed significantly (P < 0.05) compared to the control group 360 min after reperfusion. The serum AST increased gradually after reperfusion in both groups, but the AST was significantly (P < 0.05) lower in the DHP-PMX group 360 min after reperfusion.
CONCLUSION: DHP-PMX therapy reduced the hepatic warm I/R injury caused by THVE in a porcine model.
- Citation: Sato H, Oshima K, Kobayashi K, Yamazaki H, Suto Y, Takeyoshi I. Hemoperfusion with polymyxin B-immobilized fiber column improves liver function after ischemia-reperfusion injury. World J Gastroenterol 2009; 15(36): 4571-4575
- URL: https://www.wjgnet.com/1007-9327/full/v15/i36/4571.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4571
In ischemia-reperfusion (I/R), the generation of reactive oxygen species on reoxygenation inflicts tissue damage and initiates a cascade of deleterious cellular responses leading to inflammation, cell death, and ultimately organ failure[1]. Hepatic I/R injury occurs in various clinical settings, such as transplantation, trauma, liver or bowel resection, and hemorrhagic shock[2]. Severe hepatic I/R injury can lead to liver or multiple organ failure and is associated with increased morbidity and mortality[3]. Total hepatic vascular exclusion (THVE), which involves the total occlusion of the liver vasculature at the hepatoduodenal ligament (i.e. Pringle’s maneuver) and the occlusion of the inferior vena cava below and above the liver, is used during the resection of large and posterior portions of the liver clinically[4,5]. As this technique induces hepatic I/R injury, inhibition of this injury caused by THVE is necessary to obtain a better postoperative course.
The Toraymyxin polymyxin B-immobilized fiber column (PMX cartridge; Toray Industries, Tokyo, Japan) was developed in Japan in 1994 as an extracorporeal hemoperfusion device that uses polymyxin-B fixed to α-chloroacetamide-methyl polystyrene-derived fibers packed in the cartridge. Direct hemoperfusion with PMX (DHP-PMX) therapy can remove circulating endotoxin and reduce various cytokines, even in patients with high plasma cytokine levels[6]. This method has been used to treat endotoxemia[7] and was reported to lower inflammatory cytokine and plasminogen activator inhibitor-1 (PAI-1) levels immediately[8]. DHP-PMX therapy has also been found effective for severe sepsis secondary to intra-abdominal infection[9] and acute lung injury or acute respiratory distress syndrome caused by sepsis[10]. Recently, we reported the efficacy of DHP-PMX therapy in normothermic cardiopulmonary bypass[11], a pulmonary warm I/R injury model[12], and a small intestine warm I/R injury model[13].
In this study, we evaluated the usefulness of DHP-PMX therapy in warm hepatic I/R injury with a porcine THVE model.
All animals were cared for in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the guidelines set forth in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH publication 85-23, revised 1985). The study was performed under the supervision of the Animal Care and Experimental Committee of Gunma University, Showa campus, Japan.
Eleven Mexican hairless pigs (both sexes, weighing 22-38 kg) were used in this study. They were not allowed access to food for 24 h before the experiment. After administering ketamine hydrochloride (250 mg) and atropine (0.5 mg) intramuscularly, the pigs were intubated endotracheally and ventilated mechanically at a tidal volume of 25 mL/kg and a rate of 12 breaths/min. During the experiment, general anesthesia was maintained with a mixture of 1%-2% isoflurane and 100% oxygen. Lactated Ringer’s solution (20 mL/kg per hour) was infused via a catheter inserted into the right subclavian vein. A laparotomy was performed via a midline incision. The liver was skeletonized completely by dividing all of the suspensory ligaments and dissecting the retrohepatic vena cava from the posterior abdominal wall. The portal vein, hepatic artery, and common bile duct were isolated and their collaterals were occluded separately. THVE was achieved by clamping the infrahepatic and suprahepatic vena cava after clamping the portal vein and hepatic artery. An active venovenous (v-v) bypass system was started as a portosystemic shunt just before THVE to prevent congestion of the portal vein and lower body. This system consisted of a centrifugal pump system (Lifestream; St. Jude Medical, Chelmsford, MA) and venous cannulas. The blood-contact surfaces of these components were heparin-coated. The v-v bypass system was established with drains (12 Fr) inserted into the splenic and right external iliac veins for blood removal, with another drain inserted into the right external jugular vein (12 Fr) for blood return. Blood from the portal vein and infrahepatic vena cava was bypassed into the right external jugular vein via a Y-shaped shunt. The bypass blood flow was maintained at more than 20 mL/kg per minute with systemic heparinization (200 U/kg). Liver ischemia was induced by total exclusion of hepatic inflow for 120 min. After releasing the clamps to end the ischemia, the bypass system was removed. The splenic, right external iliac, and right external jugular veins were ligated after removing the cannulas. The parameters described below were measured and the animals were observed for 360 min after reperfusion.
The experimental study involved two groups: the DHP-PMX (n = 5) and control (n = 6) groups. The animals were assigned randomly to either group. In the DHP-PMX group, a double-lumen catheter was positioned in the right atrium through the left subclavian vein and DHP-PMX was performed through the catheter at a flow rate of 80 mL/min for 120 min (beginning 10 min before reperfusion). Direct hemoperfusion was not performed in the control group.
The external iliac artery was cannulated for monitoring the arterial blood pressure and collecting blood samples. Arterial blood pressure and heart rate (HR) were monitored directly through a catheter connected to a transducer (Spectramed TA 1017; San-ei, Tokyo, Japan). The rate pressure product (RPP: HR × end-systolic arterial blood pressure) was also calculated. Blood samples were collected from the same catheter before and after the procedure [before ischemia and immediately (0 min) and 30, 60, 120, 240 and 360 min after reperfusion]. All samples were centrifuged at 900 ×g for 15 min at 4°C, and the serum or plasma was frozen at -80°C for later measurement.
HTBF was measured with a laser Doppler flowmeter (Laser Blood Flow Monitor MBF 3; Moor Instruments, Devon, UK) before ischemia and immediately (0 min) and 30, 60, 120, 240 and 360 min after reperfusion. The laser probe was always placed on the right median lobe of the liver. HTBF is expressed as a percentage of the flow before ischemia.
PVBF was measured before ischemia and 30, 60, 120, 240 and 360 min after reperfusion using an electromagnetic blood flowmeter (Model MFV-3100; Nihon Kohden, Tokyo, Japan). PVBF is expressed as a percentage of the flow before ischemia.
Serum AST levels were measured at 37°C using an ultraviolet rate assay on an autoanalyzer (Hitachi 736-60; Hitachi, Tokyo, Japan) with blood samples collected and preserved using the method described above.
The results are expressed as the mean ± SE. StatView ver. 5.0 (Abacus, Berkeley, CA) was used for the statistical analyses. Statistical comparisons were made using repeated measure analysis of variance followed by Fisher’s protected least significant difference. P < 0.05 were considered to be statistically significant.
All animals survived until the endpoint of the study (360 min after reperfusion).
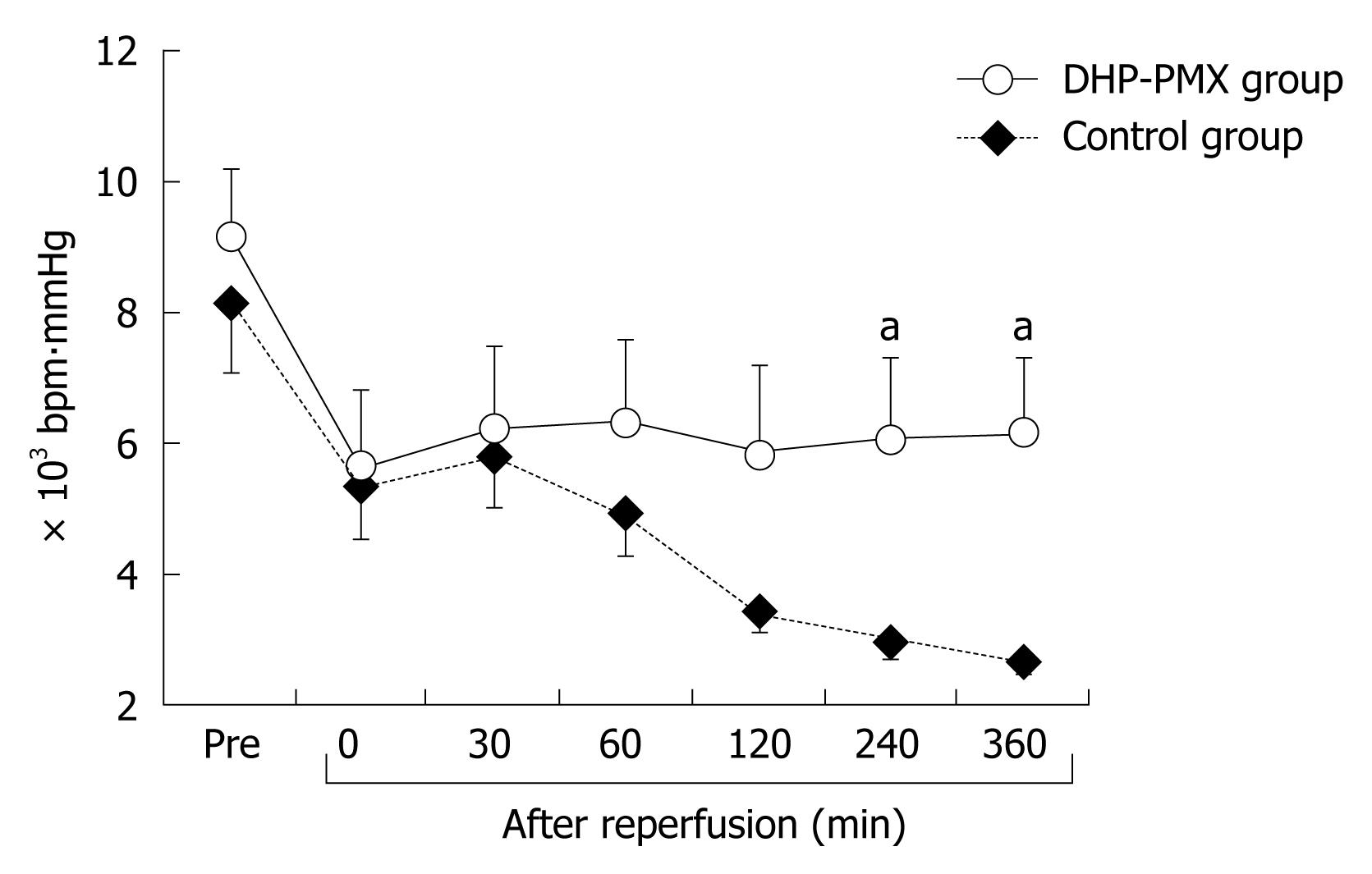
The changes in RPP were similar in both groups until reperfusion. The RPP in the control group decreased gradually until 360 min after reperfusion, while that in the DHP-PMX group was maintained at about 6000 bpm∙mmHg and differed significantly (P < 0.05) from the RPP in the control group 240 and 360 min after reperfusion (Figure 1).
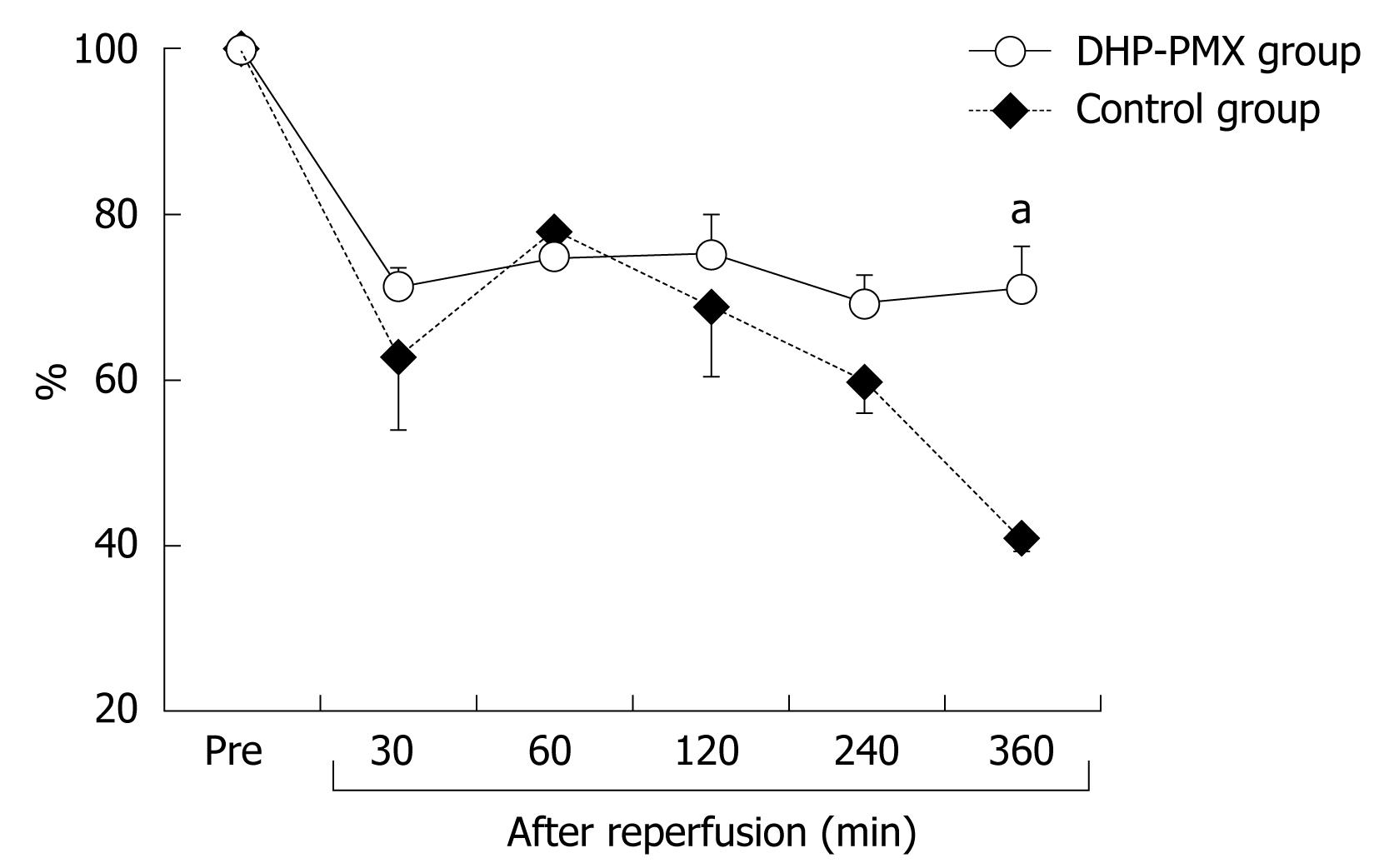
The HTBF decreased to about 20% of the baseline immediately after reperfusion in both groups (Figure 2). After reperfusion, the HTBF in the DHP-PMX group was maintained above 60% of the baseline, while no improvement was seen in the control group; the HTBF in the control group was significantly (P < 0.05) lower than that in the DHP-PMX group 240 and 360 min after reperfusion (Figure 2).
The PVBF decreased after reperfusion in both groups (Figure 3). The PVBF was similar in both groups 30 and 60 min after reperfusion. Subsequently, the PVBF in the DHP-PMX group was maintained at about 70% of the flow before ischemia, while that in the control group decreased gradually beginning 120 min after reperfusion; a significant (P < 0.05) difference was observed between the two groups 360 min after reperfusion (Figure 3).
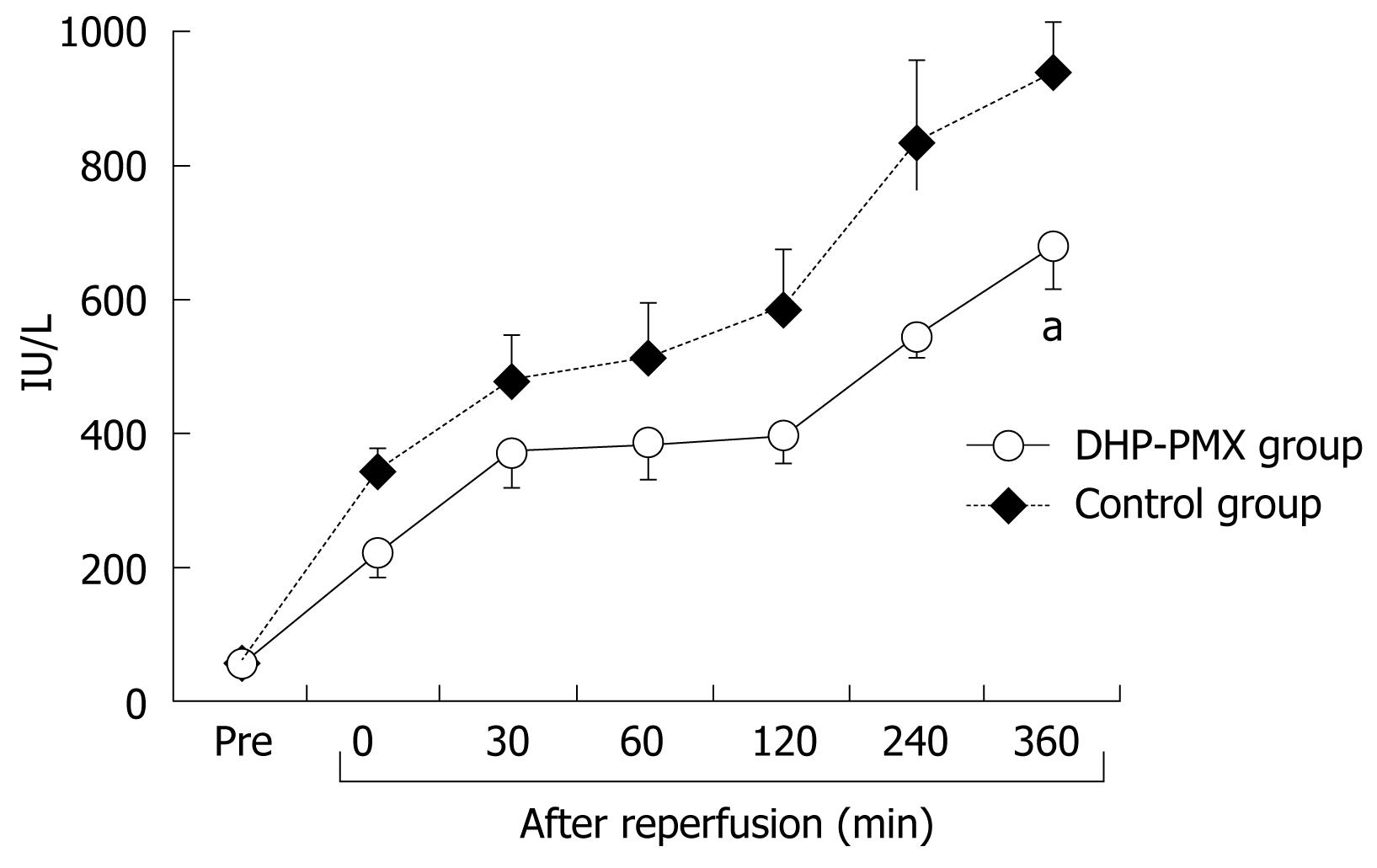
As shown in Figure 4, the serum AST before ischemia did not differ significantly between the two groups. The serum AST increased gradually after reperfusion in both groups, although the increment in the DHP-PMX group was smaller than in the control group and differed significantly 360 min after reperfusion (Figure 4).
Polymyxin B binds to endotoxin, which is an outer membrane component of Gram-negative bacteria and is thought to be an important pathogenic trigger for the production of inflammatory mediators. Several preclinical studies have demonstrated that hemoperfusion or plasmapheresis over immobilized polymyxin B removes endotoxin from blood[14-16]. Recently, some studies have shown improved hemodynamic status[17] and survival[18] in patients with sepsis who were treated with PMX. Moreover, DHP-PMX therapy was found to be effective for patients with septic shock infected with either Gram-negative or Gram-positive bacteria, which do not release endotoxins[19]. Therefore, DHP-PMX therapy is used in patients with severe sepsis or septic shock and its clinical effect has been described. In addition, studies have reported on the mechanism of DHP-PMX action. Kushi et al[20] showed that the adsorption of pathogenic bacteria prevented the release of inflammatory cytokines and reduced the stimulation of vascular endothelial cells to lower the PAI-1 level, rather than direct inhibition of PAI-1 production by DHP-PMX therapy. Tani et al[8] postulated that the reduction in plasma endotoxins by endotoxin adsorption contributed to the cessation of cytokine gene expression and the excretion of cytokines. In addition, DHP-PMX therapy improved the PaO2/FiO2 ratio in patients with acute lung injury or acute respiratory distress syndrome caused by sepsis, and this appeared to be related to decreases in the blood neutrophil elastase and IL-8 levels[10]. We hypothesized that DHP-PMX therapy might be effective in various inflammatory situations and have evaluated the utility of DHP-PMX therapy in normothermic cardiopulmonary bypass in a pig model[11], pulmonary warm I/R injury in a canine model[12], and small intestine warm I/R injury in a canine model[13] with satisfactory results. In this study, we evaluated whether DHP-PMX therapy could reduce hepatic I/R injury in a THVE model.
We found that RPP, PVBF, and HTBF in the DHP-PMX group were preserved significantly (P < 0.05) better than in the control group after reperfusion. In addition, the increase in serum AST was significantly (P < 0.05) lower in the DHP-PMX group. These results demonstrate that DHP-PMX therapy reduces the hepatic warm I/R injury caused by the THVE technique.
The natural ligands of cannabinoid receptors are lipid-like substances called endocannabinoids, and include arachidonoyl ethanolamine or anandamide and 2-arachidonoylglycerol. Cannabinoid-2 receptor agonists have been reported to have a protective effect against I/R injury in the liver and other organs by reducing endothelial cell activation, the expression of adhesion molecules such as intercellular adhesion molecule and vascular cell adhesion molecule, the levels of tumor necrosis factor-α and chemokines, neutrophil infiltration, lipid peroxidation, and apoptosis[21,22]. In addition, Wang et al[23] demonstrated that the absorption of anandamide during DHP-PMX therapy eliminated the diverse negative effects of anandamide, such as hypotension, immunosuppression, and cytotoxicity. Considering these results, DHP-PMX treatment appears not only to remove endotoxins, but also to reduce the inflammatory reaction by inhibiting various inflammatory cascades and to have an effective role in I/R injury. Further studies are required, including the suitable timing and duration and the detailed mechanisms of DHP-PMX therapy in I/R injury.
In conclusion, DHP-PMX therapy reduced the hepatic warm I/R injury caused by the THVE method using a porcine model.
Total hepatic vascular exclusion (THVE) is used during the resection of large and posterior portions of the liver clinically. This technique induces hepatic ischemia-reperfusion (I/R) injury, and severe hepatic I/R injury is associated with increased morbidity and mortality. The inhibition of the hepatic I/R injury caused by THVE is necessary to obtain a better postoperative course.
The Toraymyxin polymyxin B-immobilized fiber column (PMX cartridge) was developed as an extracorporeal hemoperfusion device. Direct hemoperfusion with PMX (DHP-PMX) therapy can remove circulating endotoxin. The authors had already demonstrated the efficacy of DHP-PMX therapy in a pulmonary warm I/R injury model and a small intestine warm I/R injury model. In this study, the authors investigated whether DHP-PMX therapy reduced the hepatic warm I/R injury caused by THVE in a porcine model.
The authors found that systemic hemodynamics, blood flow for liver and liver function were preserved significantly better in the group treated with DHP-PMX therapy than in the group with no treatment after reperfusion.
Their results demonstrate that DHP-PMX therapy reduces the hepatic warm I/R injury caused by the THVE technique.
The PMX cartridge (Toray Industries, Tokyo, Japan) was developed in Japan in 1994 as an extracorporeal hemoperfusion device that uses polymyxin-B fixed to α-chloroacetamide-methyl polystyrene-derived fibers packed in the cartridge. DHP-PMX therapy can remove circulating endotoxin and reduce various cytokines, even in patients with high plasma cytokine levels. This method has been used to treat endotoxemia and was reported to lower inflammatory cytokine and plasminogen activator inhibitor-1 levels immediately. DHP-PMX therapy has also been found effective for severe sepsis secondary to intra-abdominal infection and acute lung injury or acute respiratory distress syndrome caused by sepsis.
This is an interesting study that investigates the utility of direct hemoperfusion with a polymyxin B-immobilized fiber column (DHP-PMX therapy) on warm hepatic I/R injury with THVE using a porcine model. The title accurately reflects the major topic and contents of the study. The abstract gives a clear delineation of the research background, objectives, materials and methods, results and conclusions. Materials and Methods are very well described. The results are clearly presented and the conclusions are scientifically reliable and valuable.
Peer reviewer: Yutaka Saito, Professor, Division of Endoscopy, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. |
| 2. | Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113-118. |
| 3. | Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11:1031-1047. |
| 4. | Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13-19. |
| 5. | Delva E, Barberousse JP, Nordlinger B, Ollivier JM, Vacher B, Guilmet C, Huguet C. Hemodynamic and biochemical monitoring during major liver resection with use of hepatic vascular exclusion. Surgery. 1984;95:309-318. |
| 6. | Tsuzuki H, Tani T, Ueyama H, Kodama M. Lipopolysaccharide: neutralization by polymyxin B shuts down the signaling pathway of nuclear factor kappaB in peripheral blood mononuclear cells, even during activation. J Surg Res. 2001;100:127-134. |
| 7. | Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial. 2003;7:108-114. |
| 8. | Tani T, Hanasawa K, Kodama M, Imaizumi H, Yonekawa M, Saito M, Ikeda T, Yagi Y, Takayama K, Amano I. Correlation between plasma endotoxin, plasma cytokines, and plasminogen activator inhibitor-1 activities in septic patients. World J Surg. 2001;25:660-668. |
| 9. | Vincent JL, Laterre PF, Cohen J, Burchardi H, Bruining H, Lerma FA, Wittebole X, De Backer D, Brett S, Marzo D. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005;23:400-405. |
| 10. | Kushi H, Miki T, Okamaoto K, Nakahara J, Saito T, Tanjoh K. Early hemoperfusion with an immobilized polymyxin B fiber column eliminates humoral mediators and improves pulmonary oxygenation. Crit Care. 2005;9:R653-R661. |
| 11. | Ohki S, Oshima K, Takeyoshi I, Matsumoto K, Morishita Y. Endotoxin removal with a polymyxin B-immobilized hemoperfusion cartridge improves cardiopulmonary function after cardiopulmonary bypass. J Surg Res. 2008;145:74-79. |
| 12. | Oshima K, Akao T, Kobayashi K, Muraoka M, Matsumoto K, Takeyoshi I. The effect of direct hemoperfusion with a polymyxin B-immobilized fiber column (DHP-PMX therapy) on pulmonary ischemia-reperfusion injury in a canine model. J Invest Surg. 2008;21:127-132. |
| 13. | Sato H, Oshima K, Arakawa K, Kobayashi K, Yamazaki H, Suto Y, Takeyoshi I. Direct hemoperfusion with a polymyxin B-immobilized cartridge in intestinal warm ischemia reperfusion. World J Gastroenterol. 2008;14:5436-5441. |
| 14. | King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, Tribble CG, Kron IL. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681-1685. |
| 15. | Cohen J, Aslam M, Pusey CD, Ryan CJ. Protection from endotoxemia: a rat model of plasmapheresis and specific adsorption with polymyxin B. J Infect Dis. 1987;155:690-695. |
| 16. | Aoki H, Kodama M, Tani T, Hanasawa K. Treatment of sepsis by extracorporeal elimination of endotoxin using polymyxin B-immobilized fiber. Am J Surg. 1994;167:412-417. |
| 17. | Tetta C, Gianotti L, Cavaillon JM, Wratten ML, Fini M, Braga M, Bisagni P, Giavaresi G, Bolzani R, Giardino R. Coupled plasma filtration-adsorption in a rabbit model of endotoxic shock. Crit Care Med. 2000;28:1526-1533. |
| 18. | Uriu K, Osajima A, Hiroshige K, Watanabe H, Aibara K, Inada Y, Segawa K, Anai H, Takagi I, Ito A. Endotoxin removal by direct hemoperfusion with an adsorbent column using polymyxin B-immobilized fiber ameliorates systemic circulatory disturbance in patients with septic shock. Am J Kidney Dis. 2002;39:937-947. |
| 19. | Kawamata T, Imaizumi H, Yoshida M, Kaneko M. Polymyxin B-immobilized fiber improves hyperdynamic state in MRSA septic patients. Intensive Care Med. 1997;23:130-131. |
| 20. | Kushi H, Nakahara J, Miki T, Okamoto K, Saito T, Tanjo K. Hemoperfusion with an immobilized polymyxin B fiber column inhibits activation of vascular endothelial cells. Ther Apher Dial. 2005;9:303-307. |
| 21. | Bátkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Haskó G. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788-1800. |
| 22. | Rajesh M, Pan H, Mukhopadhyay P, Bátkai S, Osei-Hyiaman D, Haskó G, Liaudet L, Gao B, Pacher P. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol. 2007;82:1382-1389. |