Published online Feb 21, 2008. doi: 10.3748/wjg.14.1120
Revised: December 24, 2007
Published online: February 21, 2008
AIM: To analyze the difference of intestinal microbial community diversity between healthy and (S. enteritidis) orally infected ducklings.
METHODS: Enterobacterial Repetitive Intergenic Consensus (ERIC)-PCR was applied to analyze the intestinal microbial community diversity and dynamic change including duodenum, jejunum, ileum, cecum and rectum from healthy ducklings and 7-day-old ducklings after oral infection with S. enteritidis at different time points.
RESULTS: The intestinal microbial community of the control healthy ducklings was steady and the ERIC-PCR band numbers of the control healthy ducklings were the least with rectum and were the most with caecum. ERIC-PCR bands of orally inoculated ducklings did not obviously change until 24 h after inoculation (p.i.). The numbers of the ERIC-PCR bands gradually decreased from 24 h to 72 h p.i., and then, with the development of disease, the band numbers gradually increased until 6 d p.i. The prominent bacteria changed because of S. enteritidis infection and the DNAstar of staple of ERIC-PCR showed that aerobe and facultative aerobe (Escherichia coli, Shigella, Salmonella) became preponderant bacilli in the intestine of orally infected ducklings with SE.
CONCLUSION: This study has provided significant data to clarify the intestinal microbial community diversity and dynamic change of healthy and S. enteritidis orally infected ducklings, and valuable insight into the pathogenesis of S. enteritidis infection in both human and animals.
-
Citation: Cao SY, Wang MS, Cheng AC, Qi XF, Yang XY, Deng SX, Yin NC, Zhang ZH, Zhou DC, Zhu DK, Luo QH, Chen XY. Comparative analysis of intestinal microbial community diversity between healthy and orally infected ducklings with
Salmonella enteritidis by ERIC-PCR. World J Gastroenterol 2008; 14(7): 1120-1125 - URL: https://www.wjgnet.com/1007-9327/full/v14/i7/1120.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1120
Salmonella enteritidis (S. enteritidis) remains one of the main causes of food-borne illness and as such is considered to be the most important pandemic zoonosis produced under natural conditions[1–3]. It is estimated that the worldwide incidence is more than a thousand million cases, causing three million deaths each year. S. enteritidis can through the food production chain pass to humans, therefore undercooked or raw eggs and poultry meat are of a high risk for humans[45]. Infections caused by Salmonella bacteria belong to the most common zoonotic diseases worldwide[6]. Outbreaks of human salmonellosis caused by S. enteritidis have dramatically increased throughout the world since the mid-to-late 1980s and have become an important problem for the poultry industry and public health.
Intestinal microflora plays an important role in digestive function, immunity, and disease resistance. It becomes clear that intestinal resident and in-transit micro-organisms play an essential role in the homeostasis of local and systemic immunity[78]. A large number of microbial species exist in gastrointestinal tract. However, traditional culture-based techniques are inappropriate in studying the structure-function relationship of microbial communities due to their highly selective, laborious and time-consuming nature. Only a relatively small fraction of population members in a complex natural community, such as gastrointestinal system, can be recovered with culture-based techniques[9]. ERIC-PCR method developed in recent years can simultaneously compare sequence-based structural features of large numbers of community samples[10–14]. In this study, to understand the changes of intestinal microbial communities of S. enteritidis-infected ducklings, ERIC-PCR method was used to fingerprint the kinetics of microbial community of fecal samples of orally infected ducklings with S. enteritidis, which will provide valuable insight into the pathogenesis of S. enteritidis infection.
S. enteritidis (Duck, No.MY1) was isolated and maintained by Avian Disease Research Center, College of Veterinary Medicine of Sichuan Agricultural University.
The study was conducted with 68 7-day-old white Peking ducklings (the serum and feces samples of all experimental ducklings were tested and found to be S. enteritidis negative by PCR methods). All these ducklings were maintained in isolation units in a biosecure animal building and fed a commercial ducklings diet ad libitum. Two groups of ducklings were included in this study. Group 1 was used to test the dynamic change of intestinal microbial community diversity and 48 ducklings in this group were randomly divided into part A (24 orally inoculated ducklings) and part B (24 control healthy ducklings). Ducklings in part A were orally infected with 0.2 mL S. enteritidis culture solution (4 × 109 CFU/mL) and ducklings in part B were simultaneously administrated with 0.2 mL isotonic Na chloride. The clinical signs and pathological lesions of infected ducklings were monitored until 21 d after inoculation (p.i.) and the mortality was recorded in Group 2. In this group, 20 ducklings were averagely divided into two parts (C and D) at random. Ten ducklings in part C were orally infected with 0.2 mL S. enteritidis culture solution (4 × 109 CFU/mL) and 10 ducklings in part D took simultaneously 0.2 mL isotonic Na chloride.
Ducklings in Group 1 were killed and sampled at 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, 36 h, 48 h, 72 h, 6 d, 9 d p.i.. At each of the 12 sampling time points between 30 min and 9 d p.i., two ducklings of each part in Group 1 were chosen randomly for sampling. Five samples were collected from each duckling, including contents from 3 cm-long duodenum, jejunum, ileum, caecum and rectum tissues. Fresh samples were collected, put into 80% glycerol and stored at -70°C before DNA extraction.
DNA extraction procedure modified from Wei G et al[15] was used to prepare the DNA templates for PCR reactions to ensure that stable, representative and reproducible fingerprints were obtained. The sample above was suspended in 15 mL sterile 0.05 mol/L phosphate buffered saline (PBS) (1 L contained 1.48 g NaH2PO4.2H2O, 14.45 g NaH2PO4, 4.0 g NaCl; pH 7.4) followed by vortexing for 15 min in an 50-mL tube. The suspension was centrifuged at 600 ×g for 5 min and transferred to a new tube. This was repeated three times to remove coarse particles. The cells in the supernatant were collected and washed once by centrifuge at 9000 ×g for 3 min followed by resuspension in 15 mL 0.05 mol/L PBS. The cell pellets were washed three times by centrifuge at 9000 ×g for 5 min in 5 mL acetone precooling at -20°C. The washed cell pellets were collected in a new 1.5 mL tube and resuspended in 200 &mgr;L solution I (50 mmol/L Tris-base, 50 mmol/L Na2EDTA, pH 7.8), the suspension was mixed with 40 &mgr;L lysozyme solution (50 mg/mL), water-bathed for 30 min at 37°C with gentle mixing by inverting the tube every 10 min, and then was combined with 500 &mgr;L solution II (200 mmol/L NaCl, 100 mmol/L Tris-base, 50 mmol/L Na2EDTA, 2.0% SDS, 1.0% Triton X-100) and 12.5 &mgr;L Proteinase K, water-bathed for 12 h at 37°C. RNA was digested by adding RNase (0.80 g/L) at 37°C for 20 min. DNA was then purified by sequential extraction with Tris-equilibrated phenol, phenol and chloroform-isoamyl alcohol (25:24:1), and chloroform-isoamyl alcohol (24:1) followed by precipitation with two volumes of ethanol. DNA was collected by centrifugation, air dried and dissolved in 40 &mgr;L sterile TE buffer.
The total DNA samples prepared with the above protocol were characterized with agarose gel electrophoresis for integrity and size. The DNA was adjusted to 40 ng/&mgr;L and stored at -20°C before used as templates for PCR. Two &mgr;L DNA sample was added to 23 &mgr;L PCR mixtures for each PCR reaction.
Community fingerprints were obtained for intestinal microflora using total DNA as templates for ERIC-PCR. The sequence of the ERIC primers was based on work by Versalovic et al[16], E1 (ERIC1): 5’-ATGTAAGCTCCTGGGGATTCAC-3’, E2 (ERIC2): 5’-AAGTAAGTGACTGGGGTGAGCG-3’. Each 23 &mgr;L PCR reaction mixture contained 25 pmol of each primer, 5 mmol/L each deoxynucleoside triphosphate (dNTP) and 2.5 U of Taq DNA polymerase (Promega, USA) in the manufacturer’s provided buffer. Two microliters of total fecal DNA (about 80 ng) was added to reach the final 25 &mgr;L PCR reaction volume. PCR amplifications were performed in an automated thermocycler (Hybaid, USA) using the following program: 7 min at 95°C; 30 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 1 min and extension at 65°C for 8 min; followed by a final extension at 65°C for 16 min[14]. The concentrations of the PCR products were determined by DNA Quant 200 fluorometer (Pharmacia, USA) and resolved in 1.5% agarose gel electrophoresis with 300-400 ng total PCR products loaded in each lane. The gels were stained with ethidium bromide and photographed with Bio-Rad, VersaDoc Model 2000.
Gel slices containing individual major DNA bands were excised and transferred into sterile 1.5 mL Eppendorf tubes and purified by UltraClean 15 DNA Purification Kit (MO BIO, USA), according to the manufacturer’s instructions. The purified DNA was dissolved in 30 &mgr;L sterile water and the concentration was determined by DyNA Quant 200 fluorometer (Pharmacia). The purified fragments were ligated with vector pGEM-T Easy (Promega) and transformed into Escherichia coli DH5α. The transformants were plated on Luria-Bertani (LB) plates containing ampicillin and X-Gal/IPTG. The plasmids were extracted using the alkaline lysis method. For each part of experimental group, two clones were selected for sequencing (TaKaRa, Beijing). The sequences were analyzed with the BLAST program at the NCBI website (http://www.ncbi.nlm.nih.gov/blast).
Similar clinical signs and gross findings were observed in orally infected ducklings with S. enteritidis. Typical clinical signs including inappetence, depression, lameness, fervescence, and white diarrhea were observed in all infected ducklings from around 3 d p.i., and some infected ducklings had nervous symptom and tremor of head-neck. Two inoculated ducklings died as a result of S. enteritidis infection between 48 h and 60 h p.i. Necropsy revealed similar lesions in all dead ducklings, including hemorrhages of the heart, hydropericardium, exudative fibrin on the surface of the visceral pericardium, hyperaemia and haemorhage of liver. Hyperaemia and haemorhage of intestinal tract was also observed. As a result of S. enteritidis infection, 8 of 10 infected ducklings in part C of Group 2 survived and exhibited no clinical signs except growing slowly and survived throughout the infection, so the mortality of the seven-day-ducklings infected with MY1 strain of S. enteritidis was 20%.
The control healthy ducklings in part D of Group 2 had no specific clinical signs and gross findings.
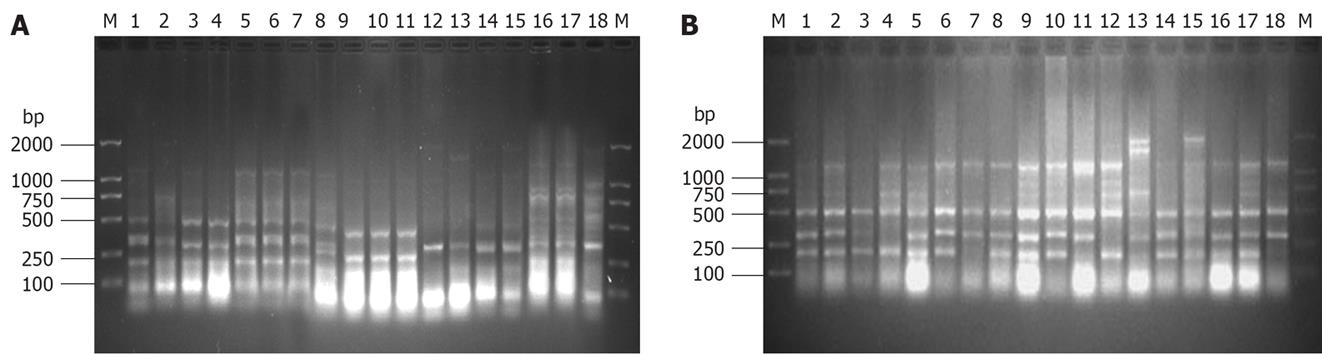
The ERIC-PCR fingerprints analysis showed that the intestinal microbial community of the control healthy ducklings in part B of Group 1 was steady. The numbers of the band formed by ERIC-PCR with rectum were the least and were the most with caecum. However, the band numbers of duodenum, ileum and jejunum between that of rectum and caecum (Figure 1A) had.
Obvious changes of ERIC-PCR bands of orally inoculated ducklings were not observed until 24 h p.i. The numbers of the ERIC-PCR bands gradually decreased from 24 h to 72 h p.i. and reached the least at 72 h p.i. Thereafter, with the development of disease, the band numbers gradually increased until 6 d p.i. Two major bands (about 300 bp and 500 bp) were detected in each sample (Figure 1B).
The sequences about 500 bp of jejunum sampled at 72 h p.i., 100 bp of duodenum sampled at 6 d p.i. of healthy ducklings and 500 bp of ileum sampled at 72 h p.i., and 300 bp of duodenum sampled at 24 h p.i. of orally infected ducklings were analyzed with the BLAST program at the NCBI website (http://www.ncbi.nlm.nih.gov/blast) (Table 1).
| Major bands of health ducklings | Major bands of infected ducklings | ||
| 100 bp (6 d duodenum) | 500 bp (72 h jejunum) | 300 bp (24 h duodenum) | 500 bp (72 h ileum) |
| Sinorhizobium meliloti enterobacterial, Pseudomonas syringae, Escherichia coli 536, UTI89, 301. (≥ 95%) | Sinorhizobium meliloti enterobacterial, Xanthomonas campestris enterobacterial, Erwinia pyrifoliae (≥ 90%) | Yersinia enterocolitica subsp, enterocolitica 8081 complete genome, Escherichia coli W3110 (APECO1, CFT073, L0001, UTI89, 536, K12-MG1655), Shigella dysenteriae Sd197, Shigella flexneri 8401 strain, Salmonella typhimurium LT2, Pseudomonas syringae pv. Phaseolicola, Pseudomonas syringae pv. glycinea, strain CYL325, Salmonella enterica subsp (≥ 90%) | Sinorhizobium meliloti enterobacterial, Xanthomonas campestris enterobacterial, Yersinia pestis (≥ 80%) |
The structural dynamics of microbial communities have been monitored using amplified sequences or total genomic DNA and a variety of genetic fingerprinting techniques, such as Terminal Fragment Length Polymorphism (T-RFLP), Amplified Ribosome DNA Restriction Analysis (ARDRA), Denaturing Gradient Gel Electrophoresis/Temperature Gradient Gel Electrophoresis (DGGE/TGGE), of amplified partial 16S/18S rDNAs or fragments of conserved functional genes and Random Amplified Polymorphic DNA (RAPD), or Arbitrarily Primed PCR (AP-PCR)[18–21]. These techniques provide genomic patterns or profiles of microbial communities, in which the number of bands reflects the number of predominant community members, while the intensity of bands theoretically reflects population levels. ERIC sequence was first described in Escherichia coli, Salmonella typhimurium and other enterobacteria[22]. Two opposing primers were designed by Versalovic et al[16] in 1991 based on the 44-bp entire conserved central core inverted repeat (ERICALL). PCR amplification of bacterial genomic DNA with this primer pair results in highly reproducible and unique banding patterns for different genomes. ERIC-PCR has been widely used for typing of bacterial genomes based on the strain-specific fingerprints. In this study, ERIC-PCR was used to fingerprint the microbial community of fecal samples of research subjects to monitor intestinal microbial community diversity and dynamic change of healthy ducklings and infected ducklings with S. enteritidis. The results indicated that intestinal microbial community diversity and dynamic change are closely correlated with the process of infection. The numbers of ERIC-PCR bands of orally inoculated ducklings gradually decreased firstly, and then, with the development of disease, the band numbers gradually increased and recovered.
A large number of microbial species exist in gastrointestinal tract. Intestinal microflora plays an important role in digestive function, immunity and disease resistance. In this study, the diversity and dynamics of intestinal microbial community did not change obviously in control healthy ducklings analyzed with ERIC-PCR, which is consistent with the study that the quantity and proportion of each kind of bacteria are steady relatively[23]. The numbers of the bands formed by ERIC-PCR were the largest in healthy caecum that contained a bacterial content of 1010 cfu/mL-1012 cfu/mL[24]. The reason why the ERIC-PCR band numbers in rectum were the least and its microbial community were not steady is that the quantity and category of microflora were mainly influenced by the proportion of bacterium in ileum and cecum[25]. Duodenum and jejunum are transition zone of intestinal tract, and bacterium would be washed away to ileum[26]. So bands of duodenum and jejunum formed by ERIC-PCR were relatively less compared with that in jejunum and caecum.
The change of intestinal internal environment can result in dysbacteriosis. So-called dysbacteriosis, is that the quantity, category and proportion of each kind of bacteria changed, which will affect the function of host’s gastrointestinal tract[27]. In this study, ERIC-PCR bands of orally inoculated ducklings did not change obviously until 24 h p.i. These results may suggest that the infection of S. enteritidis did not seriously affect intestinal microbial community of infected ducklings. However, the numbers of the ERIC-PCR bands gradually decreased from 24 h to 72 h p.i. This result may imply that intestinal microbial community diversity was affected by the continuous replication of S. enteritidis in the intestine of infected ducklings, which reflect the typical clinical sign of diarrhea and several pathological lesions of hyperaemia and haemorhage of the intestine. However, with the development of disease, the band numbers gradually increased until 6 d p.i., and the typical clinical signs and several pathological lesions also returned to almost normal at this time. Taken together, these data suggest that the stability of intestinal microbial community closely correlated with the progression of S. enteritidis infection.
Anaerobic bacterium is prominent in intestinal normal flora. In this study, the sequencing results of DNA fragments from major bands of the control healthy ducklings indicated that all these bacteria were anaerobe or facultative anaerobe and among these bacteria, Escherichia coli and Erwinia play an important role in maintaining the balance of intestinal microecology. However, few data are available in the literature showing the other bacteria with ERIC sequence were prominent bacteria in intestinal tract of fowl; further investigations are needed to clarify this. Enteric infection result in the proportion of aerobe and facultative anaerobe increase[28–30]. In this study, sequence analysis showed that the nucleotide sequence homologies of the fragment about 500 bp of infected ileum sampled at 72 h p.i. were above 80% with Sinorhizobium meliloti enterobacterial, Xanthomonas campestris enterobacterial, Yersinia pestis, and the nucleotide sequence homologies of the fragment about 300 bp of infected duodenum sampled at 24 h p.i. were more than 90% with Yersinia enterocolitica subsp, enterocolitica 8081, Escherichia coli W3110 (APEC O1, CFT073, L0001, UTI89, 536, K12-MG1655), Shigella dysenteriae Sd197, Shigella flexneri 8401 strain, Salmonella typhimurium LT2, Pseudomonas syringae pv. phaseolicola and Pseudomonas syringae pv.glycinea.strain CYL325, Salmonella enterica subsp, Erwinia amylovora. However, among these bacteria, they are all pathogenic strains able to make host dysentery except Escherichia coli K-12, MG1655. These data suggest that the pathogenic bacteria were prominent in intestinal microbial community when orally infected ducklings with S. enteritidis developed signs of diarrhea. Moreover, several pathogenic bacterial species including Yersinia pestis represent a real secondary infection, which may imply that infected ducklings with S. enteritidis were followed by secondary bacterial infection.
The lesions of intestinal mucous membrane caused by SE infection result in dysbacteriosis of intestinal normal flora, the increase of native bacterium, and the transformation from beneficial bacterium to pathogenic bacterium. Furthermore, as a result of SE infection, depression of immunity, disfunction of intestinal mucous membrane and dysbacteriosis can also raise the pathogenic bacterial levels in the intestine, such as Escherichia coli, Shigella and Salmonella. Therefore, the increased proportion of pathogenic bacteria may be crucial in determination of diarrhea symptoms in S. enteritidis infection.
Salmonella has been a concern of public health for over 100 years and the incidence of Salmonella infections has increased dramatically, especially S. enteritidis infection. Knowledge about the Salmonella infection could be an additional means for decreasing the incidence of infection. Infection with Salmonella is usually started by oral ingestion of the pathogen and is followed by dysbacteriosis of the gut and diarrhea. It is therefore, necessary to understand intestinal microbial community diversity.
Up to date, there has been no report about the intestinal microbial community diversity of animal S. enteritidis infected. ERIC-PCR, as a technique used for typing of bacterial genomes based on the strain-specific fingerprints obtained, will be an ideal method to study the intestinal microbial community diversity of S. enteritidis infected animals.
Previous studies considered that S. enteritidis established itself within the walls of the small intestine before a systemic infection could be demonstrated. However, these works were unable to identify the dynamic change of intestinal microbial community of S. enteritidis infected animals. In this study, the authors offered a significant improvement over current studies and suggested that S. enteritidis infection can cause dysbacteriosis of gastrointestinal tract.
This study will provide significant data for clarifing intestinal microbial community diversity of ducklings infected with S. enteritidis and new insight into the prevention and treatment of S. enteritidis infection.
Dysbacteriosis: The change in quantity, category and proportion of each kind of bacteria.
This study analyzed intestinal microbial community diversity of different segments of the gastrointestinal tract in healthy and orally infected ducklings with Salmonella enteritidis by ERIC-PCR method. The results show that SE infection can result in dysbacteriosis of infected ducklings, and the ERIC-PCR method is a useful tool when applied to pathogenesis and prevention of S. enteritidis infection.
| 1. | Patrick ME, Adcock PM, Gomez TM, Altekruse SF, Holland BH, Tauxe RV, Swerdlow DL. Salmonella enteritidis infections, United States, 1985-1999. Emerg Infect Dis. 2004;10:1-7. |
| 2. | Dias de Oliveira S, Siqueira Flores F, dos Santos LR, Brandelli A. Antimicrobial resistance in Salmonella enteritidis strains isolated from broiler carcasses, food, human and poultry-related samples. Int J Food Microbiol. 2005;97:297-305. |
| 3. | De Reu K, Grijspeerdt K, Messens W, Heyndrickx M, Uyttendaele M, Debevere J, Herman L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int J Food Microbiol. 2006;112:253-260. |
| 4. | Gurtler M, Fehlhaber K. Growth of Salmonella enteritidis in yolk from eggs laid by immunized hens. Int J Food Microbiol. 2004;90:107-113. |
| 5. | Gillespie IA, O'Brien SJ, Adak GK, Ward LR, Smith HR. Foodborne general outbreaks of Salmonella Enteritidis phage type 4 infection, England and Wales, 1992-2002: where are the risks? Epidemiol Infect. 2005;133:795-801. |
| 6. | Schlundt J. New directions in foodborne disease prevention. Int J Food Microbiol. 2002;78:3-17. |
| 7. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. |
| 8. | Guarner F. The intestinal flora in inflammatory bowel disease: normal or abnormal? Curr Opin Gastroenterol. 2005;21:414-418. |
| 9. | Zoetendal EG, Collier CT, Koike S, Mackie RI, Gaskins HR. Molecular ecological analysis of the gastrointestinal microbiota: a review. J Nutr. 2004;134:465-472. |
| 10. | Houf K, De Zutter L, Van Hoof J, Vandamme P. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl Environ Microbiol. 2002;68:2172-2178. |
| 11. | de la Puente-Redondo VA, del Blanco NG, Gutierrez-Martin CB, Garcia-Pena FJ, Rodriguez Ferri EF. Comparison of different PCR approaches for typing of Francisella tularensis strains. J Clin Microbiol. 2000;38:1016-1022. |
| 12. | Mehta A, Mehta YR, Rosato YB. ERIC- and REP-PCR amplify non-repetitive fragments from the genome of Drechslera avenae and Stemphylium solani. FEMS Microbiol Lett. 2002;211:51-55. |
| 13. | Ventura M, Zink R. Specific identification and molecular typing analysis of Lactobacillus johnsonii by using PCR-based methods and pulsed-field gel electrophoresis. FEMS Microbiol Lett. 2002;217:141-154. |
| 14. | Sander A, Ruess M, Bereswill S, Schuppler M, Steinbrueckner B. Comparison of different DNA fingerprinting techniques for molecular typing of Bartonella henselae isolates. J Clin Microbiol. 1998;36:2973-2981. |
| 15. | Wei G, Pan L, Du H, Chen J, Zhao L. ERIC-PCR fingerprinting-based community DNA hybridization to pinpoint genome-specific fragments as molecular markers to identify and track populations common to healthy human guts. J Microbiol Methods. 2004;59:91-108. |
| 16. | Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823-6831. |
| 17. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press 1996; 624-627. |
| 18. | Liu WT, Marsh TL, Cheng H, Forney LJ. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516-4522. |
| 19. | Marsh TL. Terminal restriction fragment length polymorphism (T-RFLP): an emerging method for characterizing diversity among homologous populations of amplification products. Curr Opin Microbiol. 1999;2:323-327. |
| 20. | Norris TB, Wraith JM, Castenholz RW, McDermott TR. Soil microbial community structure across a thermal gradient following a geothermal heating event. Appl Environ Microbiol. 2002;68:6300-6309. |
| 21. | Tannock GW. Molecular methods for exploring the intestinal ecosystem. Br J Nutr. 2002;87 Suppl 2:S199-S201. |
| 22. | Hulton CS, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5:825-834. |
| 23. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. |
| 24. | Itoh K, Narushima S. Intestinal flora of animal models of human diseases as an environmental factor. Curr Issues Intest Microbiol. 2005;6:9-15. |
| 25. | Hogberg A, Lindberg JE, Leser T, Wallgren P. Influence of cereal non-starch polysaccharides on ileo-caecal and rectal microbial populations in growing pigs. Acta Vet Scand. 2004;45:87-98. |
| 26. | Zilberstein B, Quintanilha AG, Santos MA, Pajecki D, Moura EG, Alves PR, Maluf Filho F, de Souza JA, Gama-Rodrigues J. Digestive tract microbiota in healthy volunteers. Clinics. 2007;62:47-54. |
| 27. | Rautava S, Isolauri E. The development of gut immune responses and gut microbiota: effects of probiotics in prevention and treatment of allergic disease. Curr Issues Intest Microbiol. 2002;3:15-22. |
| 28. | Liang QH, Zhang L, Duan SC, Wang P, Zhang YC, Luo JZ, Pang Y. Influence of intestinal dysbacteriosis on immune and hematopoietec function in mice. Zhonghua Erke Zazhi. 2004;42:708-711. |
| 29. | Kawai M, Matsutera E, Kanda H, Yamaguchi N, Tani K, Nasu M. 16S ribosomal DNA-based analysis of bacterial diversity in purified water used in pharmaceutical manufacturing processes by PCR and denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2002;68:699-704. |
| 30. | Christensen JE, Stencil JA, Reed KD. Rapid identification of bacteria from positive blood cultures by terminal restriction fragment length polymorphism profile analysis of the 16S rRNA gene. J Clin Microbiol. 2003;41:3790-3800. |