Published online Feb 7, 2008. doi: 10.3748/wjg.14.713
Revised: December 14, 2007
Published online: February 7, 2008
AIM: To explore the influence of polymorphisms in genes encoding for the chemokines Stromal cell-Derived Factor-1 (SDF-1)/CXCL12 and Monocyte Chemotactic Protein-1 (MCP-1)/CCL2, or for the chemokine receptor CCR5 on the risks of liver-related death and hepatocellular carcinoma (HCC) occurrence in hepatitis C virus (HCV)-infected patients.
METHODS: SDF-1 3’A, MCP-1 (-2518) and CCR5-Δ32 polymorphisms, SDF-1α, Regulated upon Activation Normal T cells Expressed and Secreted (RANTES)/CCL5 and MCP-1 serum levels were determined in 120 HCV-infected patients, included at time of cirrhosis diagnosis and prospectively followed-up.
RESULTS: During follow-up, 23/120 (19.1%) patients died and 47/120 (39.1%) developed HCC. Carriers and noncarriers of each genetic marker had similar baseline characteristics estimating the severity of liver disease. The occurrence of death or HCC during follow-up was similar among carriers and noncarriers of each polymorphism. There was no association between the carriage of mutated alleles and chemokine serum levels and the latter were not associated with the risks of death or HCC.
CONCLUSION: This study suggests the lack of association of SDF-1 3’A, MCP-1 (-2518), CCR5-Δ32 polymorphisms with death and HCC occurrence in cirrhotic HCV-infected patients.
- Citation: Nahon P, Sutton A, Rufat P, Simon C, Trinchet JC, Gattegno L, Beaugrand M, Charnaux N. Chemokine system polymorphisms, survival and hepatocellular carcinoma occurrence in patients with hepatitis C virus-related cirrhosis. World J Gastroenterol 2008; 14(5): 713-719
- URL: https://www.wjgnet.com/1007-9327/full/v14/i5/713.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.713
Host factors influencing the course of hepatitis C virus (HCV)-related liver disease are still poorly understood. As HCV infection is a major health issue worldwide leading to life-threatening complications such as hepatocellular carcinoma occurrence (HCC), refining the selection of patients with a poorer prognosis in order to intensify anti-viral therapy or HCC screening is of major interest. Among these factors, genetic polymorphisms that could either influence virological response or hepatocarcinogenesis (or both) could be in the future useful markers to improve the selection of HCV-infected patients at higher risk of developing end-stage liver diseases[1].
Chemokines constitute a large family of small (8-10 kDa) cytokines whose effects are mediated by members of a family of 7-transmembrane domain G-protein coupled receptors[2]. In the liver, chemokines have become increasingly recognized as important mediators of hepatic inflammation[3]. In particular, stromal cell-derived factor-1 (SDF-1)/CXCL12 plays a role in the recruitment and the retention of immune cells in the liver during chronic HCV and hepatitis B virus infection[4]. Lymphocytes infiltrating HCV-infected liver express high levels of the CC chemokine receptor CCR5[5] and the CCR5 ligand, regulated upon activation, normal T cells expressed and secreted (RANTES)/CCL5 may attract naive and activated T cells to the portal and periportal areas[6]. Hepatic stellate cells (HSCs) have been shown to regulate leukocyte trafficking by secreting the CC-chemokine, Monocyte Chemoattractant Protein-1 (MCP-1)/CCL2 and it has been suggested that MCP-1 may have a direct profibrogenic action via HSC chemotaxis[7].
These three major chemokines involved in the pathogenesis of inflammatory liver disease are subject to various genetic polymorphisms[3]. Several studies suggested that chemokine system polymorphisms could be involved in the clinical outcome of HCV-infected patients, either by modulating virological response or by influencing the severity of liver injury. Indeed, significant associations were found between CCR5-Δ32, which is a 32-bp deletion in the CCR5 gene leading to a nonfunctional protein[8], and reduced portal inflammation or milder fibrosis[9], as well as enhanced viral clearance[10]. Furthermore, Promrat et al suggested that expression of CCR5 and one of its ligands, RANTES, may be important in the modulation of hepatic inflammation and response to interferon therapy in chronic hepatitis C[11]. This particular CCR5-Δ32 polymorphism does not appear to be the only chemokine system genetic variant influencing the course of HCV infection; indeed, MCP-1 (-2518) allele carriage was found to be associated with higher MCP-1 secretion by hepatic stellate cells and enhanced inflammation and fibrosis[12].
Chemokines have also been shown to be involved in the development of various cancers, mainly breast carcinoma, their physiopathological action including tumour growth, invasion and metastasis[1314]. Recently, our team showed that SDF-1 stimulates human hepatoma cell growth, migration and invasion, with data obtained in human liver biopsy specimens confirming in vitro findings[15]. The homozygous (G to A) mutation at position 801 of the 3’-untranslated region of the SDF-1 gene, SDF-1 3’A has been linked to delayed progression to AIDS in adults with HIV-1 infection[16], but its influence in the course of HCV infection is still unknown.
When taking in account (1) the previously described influence of chemokine system polymorphisms on virological response, liver inflammation and fibrosis in the course of HCV infection and (2) their possible implication in various cancer development and possibly HCC, we hypothesized that these polymorphisms could influence the progression of HCV-related liver injury towards end-stage liver disease. The aim of this work was to study the influence of the most studied polymorphisms CCR5-Δ32, MCP-1 (-2518) and SDF-1 3’A on the prognosis of prospectively followed-up patients with HCV-related cirrhosis, as assessed by the risks of liver-related death and HCC occurrence. As genetic variation affecting the regulatory regions of chemokine genes may modulate their mRNA and protein levels, RANTES, SDF-1α and MCP-1 chemokine serum levels were also determined in these patients.
The present work is part of a large prospective study, which is ongoing in the department of hepatology of the Jean Verdier hospital, aiming to prospectively assess risk factors involved in HCC development in chronic liver diseases from various etiologies. Among these factors, various genetic polymorphisms have been studied over the past few years including chemokine and chemokine receptor dimorphisms[17–20].
We compiled all new HCV-infected patients who were consecutively referred to our liver unit for diagnosis and treatment between January 1, 1991 and December 31, 2001, and who fulfilled the following inclusion criteria: (1) transparietal or transjugular biopsy-proven cirrhosis; (2) chronic infection by HCV defined by positive serum HCV-RNA; (3) daily alcohol consumption < 20 g; (4) no other cause of liver disease and no infection by the human immunodeficiency virus or hepatitis B virus; (5) no evidence of HCC at the time of inclusion, as judged by negative ultrasonographic findings and serum α-fetoprotein (AFP) less than 50 ng/mL; (6) residence in France; (7) availability of a blood sample to prepare DNA; and (8) acceptance of a regular follow-up for the detection of HCC.
For each patient, the date of inclusion was the date of the first liver biopsy showing cirrhosis. Gender, age, Child-Pugh score, serum alanine aminotransferase (ALT) and aspartate transaminase (AST) levels, platelet count, HCV genotype were recorded at inclusion. The Ethics Committee of our hospital approved the trial.
Patients were prospectively evaluated every 6 mo by physical examination, ultrasonography and AFP measurements. When these investigations suggested possible HCC, computed tomography and/or magnetic resonance imaging and/or a guided liver biopsy were performed according to Barcelona criteria[21].
The two main end-points of the study were the occurrence of HCC, and the occurrence of liver transplantation or death. Follow-up ended at the date of death or liver transplantation, or at the last recorded visit (or information) within the last 6 mo before August 31, 2006, which was set as the final time limit for upgrading the patients’ file. The virological response was evaluated in patients who underwent anti-viral treatment. A sustained virological response was defined as the persistence of a negative serum HCV-RNA 6 mo after the end of treatment.
Genomic DNA was prepared from blood by standard methods[19]. All samples were recoded and blinded. Patients gave written consent for blood sampling and CCR5, SDF-1, MCP-1 genotyping. Polymerase chain reaction amplification of CCR5 alleles was performed on genomic DNA as previously described[19]. The SDF-1 3’A variant (801G to A in the 3’-untranslated region) was detected by electrophoresis on 2.5% agarose gel after PCR amplification and MspI (New England Biolabs, Saint-Quentin-en-Yvelines, France) digestion as previously described[16]. Genotype analysis for MCP-1 (-2518) was performed on genomic DNA with PCR with sequence-specific primers followed by restriction fragment length polymorphism analysis as described[2223]. Briefly, after PCR amplification, PCR products were digested with PvuII (recognizes the MCP-1 -2518 A/G transition) (New England Biolabs).
All tests were performed using frozen serum collected at fasting and stored at -80°C. SDF-1α, RANTES, MCP-1 serum levels were determined on blood samples collected at inclusion. ELISA assays were performed in patients without evidence of systemic infection at time of blood sample collection as previously described[19].
Qualitative variables were compared using the Fischer exact χ2 test or χ2 trend test with 1 degree of freedom, while quantitative variables were compared using the non-parametric Wilcoxon test.
The Kaplan-Meier method was used to estimate death and the occurrence of HCC for each parameter noted at enrolment, and the distribution of death and HCC were compared with the Log-rank test[24]. Statistical analysis used the SAS System Package version 8.02 (SAS Institute, Cary, NC).
All reported P values are two-tailed. Associations were first considered statistically significant at a two-tailed α of 0.05.
One hundred and twenty patients were included in this study. All of them had CCR5 and SDF-1 genotype assessment and 98 of them had MCP-1 genotype assessment. Demographic, biological and clinical characteristics according to the CCR5, SDF-1, and MCP-1 genotypes are summarized in Table 1. As a very small number of patients were homozygotes for CCR5-Δ32 allele (n = 0), SDF-1 3’A allele (n = 6), MCP-1 (-2518) allele (n = 6), we gathered them with heterozygotes in order to compare patients with at least one mutated allele to wild-type homozygotes.
| MCP1 genotype (n = 98) | CCR5 genotype (n = 120) | SDF-1 genotype (n = 120) | |||||||
| MCP-1/MCP-1 homozygotes | Heterozygotes or homozygotes for MCP-1 (-2518) allele | CCR5/CCR5 homozygotes | Heterozygotes or homozygotes for CCR5Δ32 allele | SDF-1/SDF-1 homozygotes | Heterozygotes or homozygotes for SDF-1 3’A allele | ||||
| (n = 56) | (n = 42) | P | (n = 106) | (n = 14) | P | (n = 65) | (n = 55) | P | |
| 57.2% | 42.8% | 88.4% | 11.6% | 54.1% | 45.9% | ||||
| Age (yr)12 | 58.7 ± 12.3 | 58.5 ± 11.8 | 0.9 | 58.7 ± 12.1 | 56.6 ± 13.1 | 0.7 | 57.3 ± 11.5 | 59.8 ± 13.0 | 0.1 |
| Male gender13 | 31 (55.3) | 17 (40.4) | 0.1 | 53 (50.0) | 8 (57.1) | 0.7 | 35 (53.8) | 25 (45.4) | 0.4 |
| ALT (× ULN)12 | 2.6 ± 1.0 | 2.9 ± 1.2 | 0.5 | 2.8 ± 1.2 | 2.2 ± 0.6 | 0.03 | 2.7 ± 1.8 | 2.8 ± 1.1 | 0.7 |
| AST (× ULN)12 | 2.1 ± 0.8 | 2.1 ± 0.7 | 0.9 | 2.2 ± 0.8 | 1.6 ± 0.6 | 0.008 | 2.1 ± 0.8 | 2.2 ± 0.9 | 0.6 |
| Platelet count (103/mm3)12 | 138.3 ± 64.7 | 142.8 ± 65.0 | 0.5 | 138.3 ± 64.7 | 142.8 ± 65.0 | 0.5 | 128.6 ± 60.1 | 152.9 ± 71.3 | 0.07 |
| Child Pugh score12 | 5.1 ± 0.6 | 5.1 ± 0.7 | 0.4 | 5.2 ± 0.7 | 5.0 ± 0.2 | 0.6 | 5.2 ± 0.8 | 5.1 ± 0.5 | 0.2 |
| MCP1 serum levels (pg/mL)12 | 448 ± 150 | 564 ± 168 | 0.9 | - | - | - | - | - | - |
| RANTES serum levels (pg/mL)12 | - | - | - | 28847 ± 18878 | 29576 ± 15905 | 0.6 | - | - | - |
| SDF-1 serum levels (pg/mL)12 | - | - | - | - | - | - | 2347 ± 690 | 2201 ± 333 | 0.7 |
| HCV genotype 13 | 40 (71.4) | 35 (83.3) | 0.2 | 82 (77.3) | 13 (92.8) | 0.2 | 49 (75.3) | 46 (83.6) | 0.3 |
| Anti-viral treatment3 | 32 (57.1) | 26 (61.9) | 0.6 | 62 (58.4) | 10 (71.4) | 0.4 | 44 67.9) | 28 (52.7) | 0.1 |
| Sustained virological3 Response | 14 (25.0) | 11 (26.1) | 0.9 | 25 (23.5) | 3 (21.4) | 0.9 | 16 (24.6) | 12 (21.8) | 0.8 |
| HCC development3 | 17 (30.3) | 19 (45.2) | 0.1 | 43 (40.5) | 4 (28.5) | 0.5 | 26 (40.0) | 21 (38.1) | 0.7 |
| Death3 | 10 (17.8) | 6 (14.2) | 0.7 | 20 (18.8) | 3 (21.4) | 0.7 | 11 (16.9) | 12 (21.8) | 0.4 |
Baseline characteristics estimating the severity of liver disease (prothrombin time, bilirubin levels, albumin, ascites, encephalopathy, Child-Pugh score) were similar among carriers and noncarriers of each genetic marker (Table 1 and data not shown). Demographic data were not different according to allele distributions. Finally, we did not observe any association between the studied chemokine system polymorphisms and the corresponding baseline chemokine serum levels. Thus, there was no association between the carriage of CCR5-Δ32 and the serum levels of RANTES, neither between the carriage of SDF-1 3’A and SDF-1α serum levels, nor between MCP-1 (-2518) allele carriage and MCP-1 serum levels.
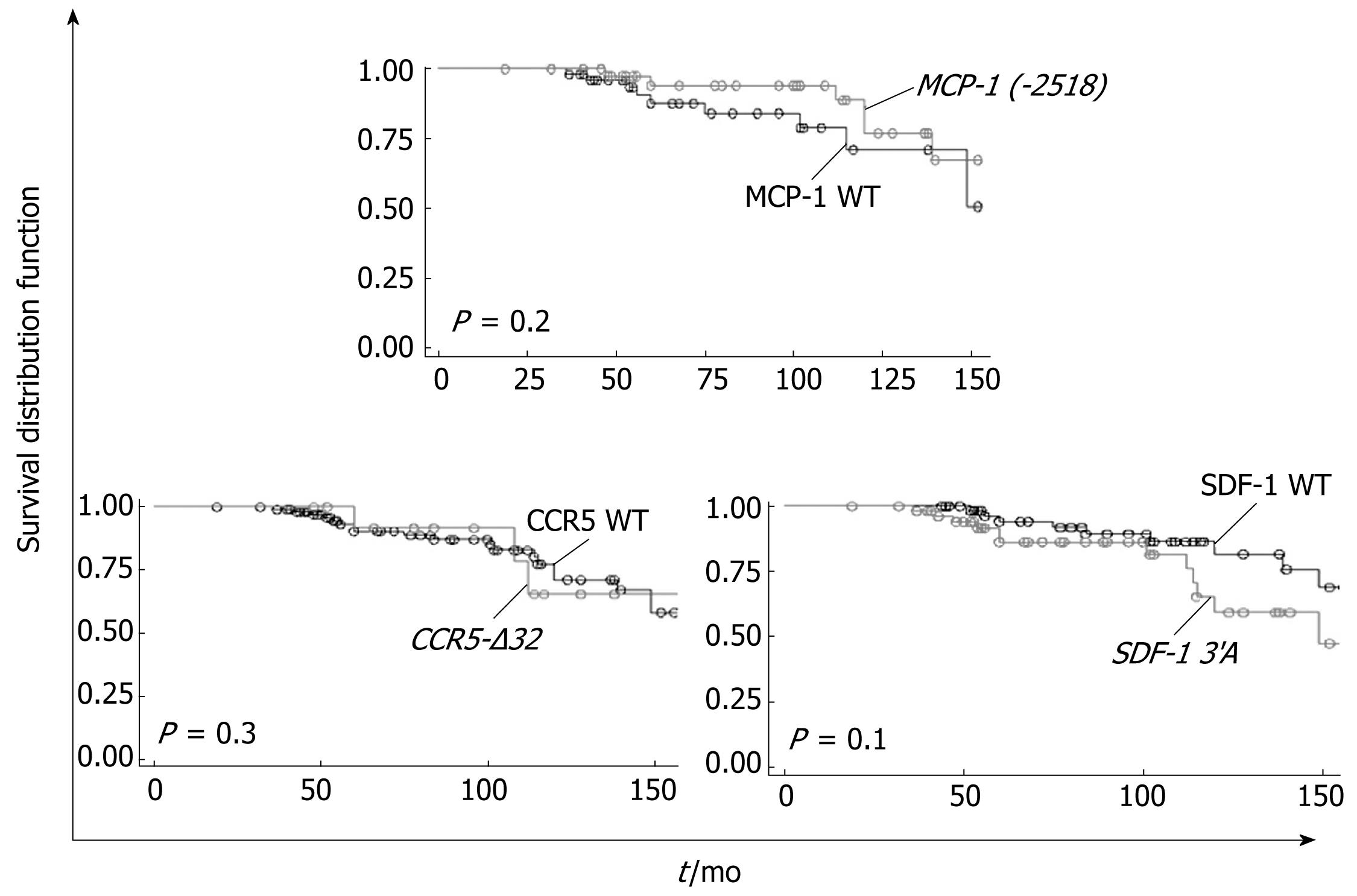
The incidence of death or transplantation among carriers and noncarriers of each polymorphism is shown in Table 1. Figure 1 displays their prospective influence on these events.
During follow-up, 23/120 (19.1%) patients died or underwent liver transplantation (n = 2). Death was attributable to liver disease in all cases, due to advanced HCC in 19 cases or due to variceal bleeding and/or liver failure in 4 cases. None of the patients included in this study were lost during follow-up. Mean time of follow-up of the cohort was 90.7 ± 43.2 mo.
The incidence of death or transplantation during follow-up was similar among carriers and noncarriers of each genetic marker (Table 1). Using the Kaplan-Meier method, we successively studied the influence of the mutated alleles as risk factors for death/liver transplantation in this cohort. None of the studied polymorphisms had an influence on these events (Figure 1). According to genotypes, quartile time of survival was 112 mo in patients carrying at least one allele CCR5-Δ32 vs 120 mo in wild-type homozygotes [RR = 1.0 (95% CI = 0.3-3.4), P = 0.3]; 114 mo in patients carrying at least one allele SDF-1 3’A vs 149 mo in wild-type homozygotes [RR = 1.9 (95% CI = 0.8-4.4), P = 0.1]; 139 mo in patients carrying at least one allele MCP-1 (-2518) vs 115 mo in wild-type homozygotes [RR = 0.5 (95% CI = 0.1-1.5), P = 0.2].
Seventy-two/120 patients underwent curative anti-viral therapy during follow-up. According to genotype distribution, the proportion of treated patients was similar in each group, as well as the infection by HCV genotype 1 which was the most represented in the cohort. None of the studied polymorphisms influenced the sustained virological response (SVR) (Table 1).
Finally, baseline RANTES, SDF-1α or MCP-1 serum levels were not associated with the risk of death/transplantation in our cohort or with the probability of SVR (data not shown).
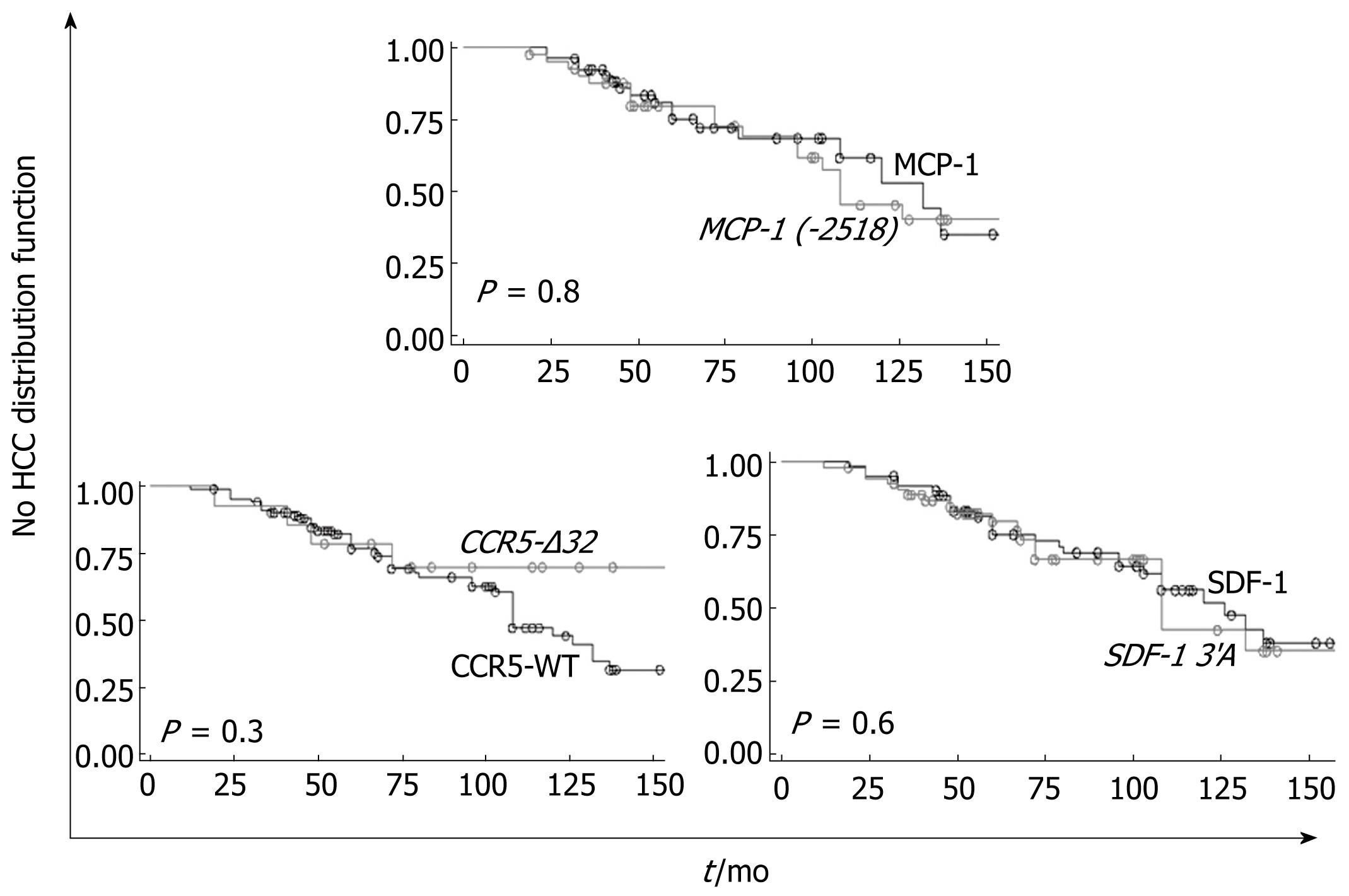
Although inclusion criteria required the absence of detectable HCC at the time of inclusion, 47/120 (39.1%) patients developed HCC during follow-up. Mean time to occurrence of HCC in this cohort was 78.5 ± 40.5 mo.
HCC occurrence during follow-up was not different among carriers and noncarriers of each polymorphism (Table 1). Using the Kaplan-Meier method, we successively studied the influence of the mutated alleles as risk factors for HCC development in this cohort. None of the studied polymorphisms had an influence on this event (Figure 2). According to genotypes, quartile time to HCC occurrence was 72 mo in patients carrying at least one allele CCR5-Δ32 vs 68 mo in patients wild-type homozygotes [RR = 0.5 (95% CI = 0.2-1.6), P = 0.3]; 72 mo in patients carrying at least one allele SDF-1 3’A vs 68 mo in wild-type homozygotes [RR = 1.1 (95% CI = 0.6-2.0), P = 0.6]; 67 mo in patients carrying at least one allele MCP-1 (-2518) vs 72 mo in patients wild-type homozygotes [RR = 1.1 (95% CI = 0.5-2.1), P = 0.8].
Finally, baseline RANTES, SDF-1α or MCP-1 serum levels were not associated with the risk of HCC occurrence in our cohort (data not shown).
The results of this study are consistent with a lack of influence of CCR5-Δ32, SDF-1 3’A and MCP-1 (-2518) chemokine system polymorphisms on the prognosis of patients with HCV-related cirrhosis as observed in patients with alcoholic cirrhosis[19]. Our hypothesis, based on the results of several case-control studies displaying an involvement of these genetic variants in the progression of liver injury in HCV-infected patients, was tested on a large cohort of prospectively followed-up patients with a large number of events allowing us to be confident in such a conclusion. The observed discrepancies between the present work and the previously published studies could have several explanations[9101225].
Case-control studies are subject to many methodological biases, including selection bias. Some controversial results have already been reported regarding the involvement of chemokine system polymorphisms in the course of HCV infection. Indeed, Woitas et al reported a higher prevalence of the CCR5-Δ32 allele carriage in HCV-infected patients compared with controls, suggesting that this genetic variant could be associated with a higher risk of developing chronic infection when exposed to the virus[25]. This finding has never been confirmed[9–1126], raising the issue of selection of specific sub-groups of patients displaying different levels of HCV exposure and the need to confirm such results in independent cohorts. Nevertheless, despite these methodological limitations and controversies[2527] common to all case-control studies, the influence of chemokine system polymorphisms on HCV-related liver injury relies on solid histological and biological data obtained in human biopsy specimen[9–11].
Our main end-points were the occurrence of liver-related death and HCC in our cohort. These events depend on various and numerous factors, including therapeutic management that can modify the natural course of the disease. As a matter of fact, active preventive procedures aiming to lower life-threatening complications have been carried out in this cohort such as digestive hemorraghe prevention by band ligation or interferon therapy which has been shown to improve survival and lower the risk of HCC incidence in patients with HCV-related cirrhosis[2829]. As well, as regular HCC screening was carried out in our cohort, patients with small HCC underwent curative treatment, thus modifying their prognosis. Despite the prospective aspect of this study, one could wonder if such therapeutic interventions could induce major changes in the natural course of the disease, thus modifying the importance of chemokine system involvement on the progression of liver injury.
A secondary aim of this work was to assess the influence of the studied polymorphisms on anti-viral therapy. Indeed, several reports showed that CCR5 polymorphisms could be host genetic factors predicting anti-viral treatment success, suggesting that this chemokine receptor could be involved in interferon therapy response[113031]. In the present paper, we did not observe any influence of the studied polymorphisms on anti-viral treatment response.
The functional consequences of the studied polymorphisms are still poorly understood, requiring further in vitro and in vivo studies aiming to assess if chemokines may be fair serum host factors to explain the inter-individuals differences of evolutions in HCV-infected patients. No association was found between the polymorphisms under study and the baseline serum levels of the corresponding chemokines. Furthermore, these serum levels did not influence the outcome of patients. Nevertheless, the selection of cirrhotic patients is unlikely to be a good choice for the study of the association of polymorphisms with serum levels of a given compound as liver function might affect circulating levels. The assessment of the correlation between chemokine system genetic variants and the corresponding circulating chemokines as well as their possible influence on hepatic injury should be conducted in patients without liver function impairment.
However, these data do not exclude that the chemokine levels in the liver may vary during the course of HCV-related liver disease, as demonstrated by others[32]. Indeed, a statistically significant association between the intrahepatic RANTES expression and the inflammatory activity of chronic hepatitis C was found[32].
It seems reasonable to consider that the progression of liver injury in the course of HCV infection is a continuous pathological process from primo-infection to the development of end-stage liver disease. Nevertheless, mechanisms involved in this progression may not have the same implication before and after the onset of cirrhosis. Thus, the influence of chemokine system could be therefore more critical during the first steps of the infection during which liver inflammation and fibrogenesis are the main physiopathological events. Conversely, their involvement in hepatocarcinogenesis or the progression of liver injury towards liver failure and portal hypertension may not be significant enough (or such events the consequences of too many pathological pathways) to observe an influence of their genetic variants.
In conclusion, the results of this study display a lack of influence of major chemokine system polymorphisms towards liver-related death and HCC occurrence that were previously described as possible host factors influencing the course of HCV infection. This finding highlights the need to assess the prognostic value of such polymorphisms in prospectively followed-up cohorts of patients. If confirmed by other independent cohort studies as previously reported[27], these results suggest that CCR5-Δ32, SDF-1 3’A and MCP-1 (-2518) polymorphisms are not fair candidate genetic variants to select HCV-infected patients at higher risk of developing end-stage liver disease.
Hepatitis C virus (HCV)-related cirrhosis is a life threatening disease with annual incidences of hepatocellular carcinoma (HCC) and death reaching around 4% and 3% respectively. However, there is wide variability in susceptibility to HCV-related cirrhosis and its outcome such as HCC or death. As epidemiologic factors leading to these complications are well established, the genetic background underlying these differences is still poorly understood and relies on the associations of genetic polymorphisms with such events.
Polymorphisms in genes encoding for chemokines or chemokine receptors have been associated with the progression of HCV-related liver injury and with various cancer development. However, most of published works are conducted in small-size populations and are case-control studies, thus limiting the reliability of the results observed and the conclusions drawn. Furthermore, their influence on the risks of liver-related death and HCC occurrence in HCV-infected patients is still unknown.
The results of this study are consistent with a lack of influence of chemokine system polymorphisms on the prognosis of patients with HCV-related cirrhosis. This study was carried out in a large cohort of prospectively followed-up patients with a large number of events allowing us to be confident in such a conclusion.
Exploring new gene variants associated with HCV cirrhosis outcome could be in the future useful markers to improve the selection of HCV-infected patients at higher risk of developing end-stage liver disease.
HCC is the most frequent primary liver cancer and the first cause of death in patients with HCV-cirrhosis.
This paper shows clearly the lack of any relationship between several genetic markers, the serum level of cytokines and the prognosis of HCV related HCC. Despite their negative results, the study is very well done and described.
| 1. | Koike K, Tsutsumi T, Fujie H, Shintani Y, Kyoji M. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62 Suppl 1:29-37. [Cited in This Article: ] |
| 2. | Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121-127. [Cited in This Article: ] |
| 3. | Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899-d1914. [Cited in This Article: ] |
| 4. | Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, Franitza S, Weiss I, Avniel S, Boaz P. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164-1174. [Cited in This Article: ] |
| 5. | Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236-6243. [Cited in This Article: ] |
| 6. | Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415-422. [Cited in This Article: ] |
| 7. | Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140-148. [Cited in This Article: ] |
| 8. | Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722-725. [Cited in This Article: ] |
| 9. | Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, Satsangi J, Wright M, Zhang L, Thomas HC. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468-1476. [Cited in This Article: ] |
| 10. | Goulding C, McManus R, Murphy A, MacDonald G, Barrett S, Crowe J, Hegarty J, McKiernan S, Kelleher D. The CCR5-delta32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut. 2005;54:1157-1161. [Cited in This Article: ] |
| 11. | Promrat K, McDermott DH, Gonzalez CM, Kleiner DE, Koziol DE, Lessie M, Merrell M, Soza A, Heller T, Ghany M. Associations of chemokine system polymorphisms with clinical outcomes and treatment responses of chronic hepatitis C. Gastroenterology. 2003;124:352-360. [Cited in This Article: ] |
| 12. | Muhlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Scholmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085-1093. [Cited in This Article: ] |
| 13. | Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143-154. [Cited in This Article: ] |
| 14. | Manes S, Mira E, Colomer R, Montero S, Real LM, Gomez-Mouton C, Jimenez-Baranda S, Garzon A, Lacalle RA, Harshman K. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J Exp Med. 2003;198:1381-1389. [Cited in This Article: ] |
| 15. | Sutton A, Friand V, Brule-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poire A, Saffar L, Kraemer M, Vassy J. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21-33. [Cited in This Article: ] |
| 16. | Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, Dean M, Honjo T, Tashiro K, Yabe D. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC). Science. 1998;279:389-393. [Cited in This Article: ] |
| 17. | Nahon P, Sutton A, Pessayre D, Rufat P, Degoul F, Ganne-Carrie N, Ziol M, Charnaux N, N’kontchou G, Trinchet JC. Genetic dimorphism in superoxide dismutase and susceptibility to alcoholic cirrhosis, hepatocellular carcinoma, and death. Clin Gastroenterol Hepatol. 2005;3:292-298. [Cited in This Article: ] |
| 18. | Sutton A, Nahon P, Pessayre D, Rufat P, Poire A, Ziol M, Vidaud D, Barget N, Ganne-Carrie N, Charnaux N. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol-induced cirrhosis. Cancer Res. 2006;66:2844-2852. [Cited in This Article: ] |
| 19. | Nahon P, Sutton A, Rufat P, Faisant C, Simon C, Barget N, Trinchet JC, Beaugrand M, Gattegno L, Charnaux N. Lack of association of some chemokine system polymorphisms with the risks of death and hepatocellular carcinoma occurrence in patients with alcoholic cirrhosis: a prospective study. Eur J Gastroenterol Hepatol. 2007;19:425-431. [Cited in This Article: ] |
| 20. | Nahon P, Sutton A, Pessayre D, Rufat P, Ziol M, Ganne-Carrie N, Charnaux N, Trinchet JC, Gattegno L, Beaugrand M. Manganese superoxide dismutase dimorphism and iron overload, hepatocellular carcinoma, and death in hepatitis C virus-infected patients. Clin Gastroenterol Hepatol. 2007;5:630-635. [Cited in This Article: ] |
| 21. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [Cited in This Article: ] |
| 22. | Szalai C, Duba J, Prohaszka Z, Kalina A, Szabo T, Nagy B, Horvath L, Csaszar A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp (a) and MCP-1 -2518 G/G genotype in CAD patients. Atherosclerosis. 2001;158:233-239. [Cited in This Article: ] |
| 23. | Hizawa N, Yamaguchi E, Furuya K, Jinushi E, Ito A, Kawakami Y. The role of the C-C chemokine receptor 2 gene polymorphism V64I (CCR2-64I) in sarcoidosis in a Japanese population. Am J Respir Crit Care Med. 1999;159:2021-2023. [Cited in This Article: ] |
| 24. | Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [Cited in This Article: ] |
| 25. | Woitas RP, Ahlenstiel G, Iwan A, Rockstroh JK, Brackmann HH, Kupfer B, Matz B, Offergeld R, Sauerbruch T, Spengler U. Frequency of the HIV-protective CC chemokine receptor 5-Delta32/Delta32 genotype is increased in hepatitis C. Gastroenterology. 2002;122:1721-1728. [Cited in This Article: ] |
| 26. | Ruiz-Ferrer M, Barroso N, Antinolo G, Aguilar-Reina J. Analysis of CCR5-Delta 32 and CCR2-V64I polymorphisms in a cohort of Spanish HCV patients using real-time polymerase chain reaction and fluorescence resonance energy transfer technologies. J Viral Hepat. 2004;11:319-323. [Cited in This Article: ] |
| 27. | Tommasi AM, Fabris P, Carderi I, Baragiotta A, Baldo V, Venturi C, Giordani MT, Tositti G, Floreani A. Lack of higher frequency of the chemokine receptor 5-delta32/delta32 genotype in hepatitis C. J Clin Gastroenterol. 2006;40:440-443. [Cited in This Article: ] |
| 28. | Heathcote EJ. Prevention of hepatitis C virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S294-S302. [Cited in This Article: ] |
| 29. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [Cited in This Article: ] |
| 30. | Ahlenstiel G, Berg T, Woitas RP, Grunhage F, Iwan A, Hess L, Brackmann HH, Kupfer B, Schernick A, Sauerbruch T. Effects of the CCR5-Delta32 mutation on antiviral treatment in chronic hepatitis C. J Hepatol. 2003;39:245-252. [Cited in This Article: ] |
| 31. | Konishi I, Horiike N, Hiasa Y, Michitaka K, Onji M. CCR5 promoter polymorphism influences the interferon response of patients with chronic hepatitis C in Japan. Intervirology. 2004;47:114-120. [Cited in This Article: ] |
| 32. | Apolinario A, Majano PL, Alvarez-Perez E, Saez A, Lozano C, Vargas J, Garcia-Monzon C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861-2870. [Cited in This Article: ] |