Published online Dec 28, 2008. doi: 10.3748/wjg.14.7353
Revised: November 3, 2008
Accepted: November 10, 2008
Published online: December 28, 2008
AIM: To investigate the protective effects of magnolol on sepsis-induced inflammation and intestinal dysmotility.
METHODS: Sepsis was induced by a single intraperitoneal injection of lipopolysaccharide (LPS). Male Wistar rats were randomly assigned to one of three treatment groups: magnolol prior to LPS injection (LPS/Mag group); vehicle prior to LPS injection (LPS/Veh group); vehicle prior to injection of saline (Control/Veh). Intestinal transit and circular muscle mechanical activity were assessed 12 h after LPS injection. Tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), monocyte chemoattractant protein-1 (MCP-1) and inducible nitric oxide synthase (iNOS) mRNA in rat ileum were studied by RT-PCR 2 h after LPS injection. Nuclear factor-κB (NF-κB) activity in the intestine was also investigated at this time using electrophoretic mobility shift assay. In addition, antioxidant activity was determined by measuring malondialdehyde (MDA) concentration and superoxide dismutase (SOD) activity in the intestine 2 h after LPS injection.
RESULTS: Magnolol significantly increased intestinal transit and circular muscle mechanical activity in LPS-treated animals. TNF-α, MCP-1 and iNOS mRNA expression in the small intestine were significantly reduced after magnolol treatment in LPS-induced septic animals, compared with untreated septic animals. Additionally, magnolol significantly increased IL-10 mRNA expression in septic rat ileum. Magnolol also significantly suppressed NF-κB activity in septic rat intestine. In addition, magnolol significantly decreased MDA concentration and increased SOD activity in rat ileum.
CONCLUSION: Magnolol prevents sepsis-induced suppression of intestinal motility in rats. The potential mechanism of this benefit of magnolol appears to be modulation of self-amplified inflammatory events and block of oxidative stress in the intestine.
- Citation: Yang TC, Zhang SW, Sun LN, Wang H, Ren AM. Magnolol attenuates sepsis-induced gastrointestinal dysmotility in rats by modulating inflammatory mediators. World J Gastroenterol 2008; 14(48): 7353-7360
- URL: https://www.wjgnet.com/1007-9327/full/v14/i48/7353.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7353
| Gene | Primer sequences | Product size (bp) |
| β-actin | F 5' GAAATCGTGCGTGACATTA 3' | 349 |
| R 5' TAGGAGCCAGGGCAGTAA 3' | ||
| TNF-α | F 5' GTAGCAAACCACCAAGCAG 3' | 211 |
| R 5' GGTATGAAATGGCAAATCG 3' | ||
| IL-10 | F 5' GCTATGTTGCCTGCTCTT 3' | 307 |
| R 5' ATGCTCCTTGATTTCTGG 3' | ||
| MCP-1 | F 5' ACTTGACCCATAAATCTGA 3' | 168 |
| R 5' TGGAAGGGAATAGTGTAAT 3' | ||
| iNOS | F 5' TTGGGTCTTGTTAGCCTAGTC 3' | 264 |
| R 5' TGTGCAGTCCCAGTGAGGAAC 3' |
Sepsis frequently occurs after trauma, burns, hemorrhage or abdominal surgery. It is a leading cause of morbidity and mortality in critically ill patients[1]. During sepsis, the most frequent complications within the gastrointestinal (GI) tract are ileus and mucosal barrier dysfunction[2]. Ileus plays an important role in the pathophysiology of sepsis by promoting bacterial stasis, bacterial overgrowth and bacterial translocation, which lead to the development of secondary infections and multiple organ failure[3].
Although common during sepsis, the etiology of ileus is still unclear. Current evidence supports the hypothesis that lipopolysaccharide (LPS) rapidly activates resident intestinal macrophages, which subsequently initiate a molecular and cellular inflammatory response that causes intestinal dysmotility[4-6]. Additionally, oxidative stress during sepsis may also be involved in this process[7]. Currently, there is no accepted pharmacological prevention or management of sepsis-induced intestinal dysmotility. Blocking oxidative stress and modulating the inflammatory events might be helpful.
Magnolia officinalis, a traditional Chinese herb, is commonly used in the treatment of abdominal distention and vomiting associated with many clinical conditions. It has been reported to attenuate L-arginine-induced GI dysmotility in rodents[8], and to improve the electrical activity of GI smooth muscle during endotoxemia[9]. Recently, magnolol (5,5’-di-2-propenyl-1,1’-biphenyl-2,2’-diol), a principal constituent isolated from the bark of Magnolia officinalis, has been showed to attenuate peroxidative damage and to improve survival of rats with sepsis[10]. Treatment with magnolol after hemorrhagic shock can suppress the tumor necrosis factor-α (TNF-α) level and preserve interleukin-10 (IL-10) production in rats[11].
Thus, we have developed the hypothesis that through modulation of inflammatory cytokines during sepsis, magnolol may be helpful for treatment of sepsis-induced ileus. Therefore, the objective of the present study was to examine the capacity of magnolol pretreatment to prevent sepsis-induced intestinal dysmotility and to determine its effects on pro- and anti-inflammatory molecular responses in the local intestine.
Male Wistar rats (250-300 g body weight) were obtained from the Academy of Military Medicine Sciences (Beijing, China). The rats were exposed to 12 h light and 12 h darkness each day, with free access to food and water. All experiments were performed in accordance with the institutional criteria for the care and use of laboratory animals in research. Sepsis was induced by a single intraperitoneal injection of LPS (Escherichia coli, O55: B5; Sigma, St Louis, MO, USA) at 20 mg/kg. Controls received intraperitoneal injections of saline.
Magnolol (National Institute for the Control of Pharmaceutical and Biological Products, China) was dissolved in 40% (v/v) propylene glycol and diluted to the desired concentration in normal saline. Final concentration of propylene glycol in the injected solution was < 4.0 × 10-3% (v/v). The single dose used for the magnolol instillation was 10-5 g/kg, which was previously shown to be helpful for increasing survival of surgically induced sepsis[10]. Normal saline with 4.0 × 10-3% (v/v) propylene glycol served as a vehicle.
Animals were randomly assigned to one of three treatment groups. LPS/Mag group: rats received magnolol (10-5 g/kg, intravenous bolus via the tail vein) 30 min before LPS injection; LPS/Veh group: rats received vehicle 30 min before LPS injection; Control/Veh: rats received vehicle 30 min before injection of saline. Preliminary results showed that intraperitoneal injection of LPS caused a profound suppression of intestine muscle contractile activity, which was both dose- and time-dependent. Furthermore, the effects of LPS are always rat strain specific and relate to the serotype of LPS[12]. In this study, we chose the 12-h time point for measurement of intestinal smooth muscle function. To elucidate the potential mechanism for magnolol preventing sepsis-induced ileus, we also evaluated changes in the chemokines and cytokines in the intestine 2 h after LPS injection, because the inflammatory response in the local intestine rapidly initiated by LPS is always responsible for GI dysmotility[4-6].
Twelve hours after LPS (or saline) was administered, the animals received an intragastric injection of 0.1 mL Evans blue (50 mg in 1 mL 0.9% NaCl). Then, the rats were killed by exsanguination 1 h later. Intestinal transit was determined by measuring the distance between the gastric pylorus and distal small intestine that was stained blue[13].
Circular muscle mechanical activity was assessed using full-thickness strips obtained from the ileum of each animal 12 h after LPS (or saline) injection. Muscle strips (2 × 10 mm) were placed in a mechanical organ chamber that was continuously perfused with pre-oxygenated Krebs-bicarbonate solution maintained at 37°C. One end of each strip was tied to a fixed post, and the other was attached to an isometric force transducer. After an equilibration period of 30 min, spontaneous mechanical contractions were recorded. The contractile responsiveness of muscle strips to the muscarinic receptor agonist bethanechol was also evaluated. Dose-response curves were generated by exposing the muscles to increasing concentrations of bethanechol (0.1-100 μmol/L) for 10 min; with intervening 20-min wash periods. Contractions were recorded, measured, and stored in a computer using a commercially available hardware and software package (TaiMeng Technology, Chengdu, China).
To elucidate the potential mechanism of magnolol treatment blunting sepsis-induced intestinal dysmotility, mRNA for TNF-α, IL-10, monocyte chemoattractant protein 1 (MCP-1) and inducible nitric oxide synthase (iNOS) in rat ileum was assessed by RT-PCR.
Total mRNA was extracted with TRIZOL Reagent (GIBCO BRL, USA). Reverse transcription was performed using a Reverse Transcription System Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Primers were designed and purchased from AuGCT Biotechnology (Beijing, China). β-actin was used as an endogenous control. The sequences of the RT-PCR primers are listed in Table 1. PCR was performed with 25 μL reaction mixture of 1 μL RT product, 2 mmol/L MgCl2, 0.03 U/L Taq DNA polymerase, 0.4 mmol/L dNTP, 0.1 μmol/L primer (endogenous control, target genes), and 1 × Taq DNA polymerase magnesium-free buffer. Then, the reaction mixture was overlaid with two drops of mineral oil and incubated in a thermocycler (Eppendorf, Germany) programmed to pre-denature at 94°C for 2 min, denatured at 94°C for 30 s, annealed at 55°C for 30 s, and extended at 72°C for 30 s for a total of 30 cycles. The last cycle was followed by a final incubation at 72°C for 6 min and cooling to 4°C. PCR products were electrophoresed on a 1.2% agarose gel and saved as digital images. Relative quantities of target gene mRNA were analyzed by Quantity One software (Bio-Rad Laboratories, USA), normalized with β-actin expression.
Nuclear protein of rat ileum was prepared by hypotonic lysis followed by high salt extraction[14,15]. Nuclear factor-κB (NF-κB) activity in the nuclear extract was analyzed using the EMSA kit according to the manufacturer’s protocol (Gel Shift Assay System; Promega). In brief, an NF-κB oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was end-labeled with [γ-32P] ATP and T4-polynucleotide kinase. Binding assays were performed in 10 μL binding reaction mixture that contained 10 μg nuclear proteins and [γ-32P]-labeled NF-κB oligonucleotides. The binding reaction mixture was incubated at room temperature for 20 min and then electrophoresed on 4% non-denaturing PAGE. After PAGE, the gels were dried and exposed to X-ray film. The autoradiograms were quantified by scanning densitometry, using Quantity One software (Bio-Rad US).
To evaluate the antioxidative capacity of magnolol, MDA concentration and SOD activity in rat ileum were measured 2 h after LPS injection. Intestinal tissue samples were thawed, weighed and homogenized 1:9 (w/v) in 0.9% saline. The homogenates were centrifuged at 3000 r/min for 10 min at 4°C, and the supernatant was removed for the assay of MDA content, SOD activity and total protein.
Total intestinal protein concentration was determined using the Coomassie blue method, with bovine serum albumin as a standard. SOD activity and MDA level were detected with kits, according to the manufacturer’s instructions (Jiancheng Bioengineering Ltd, Nanjing, China). Results were expressed as N/mg protein and nmol/mg protein, respectively.
Data were expressed as mean ± SE. Statistical significance was determined by one-way ANOVA using SPSS 11.0 (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
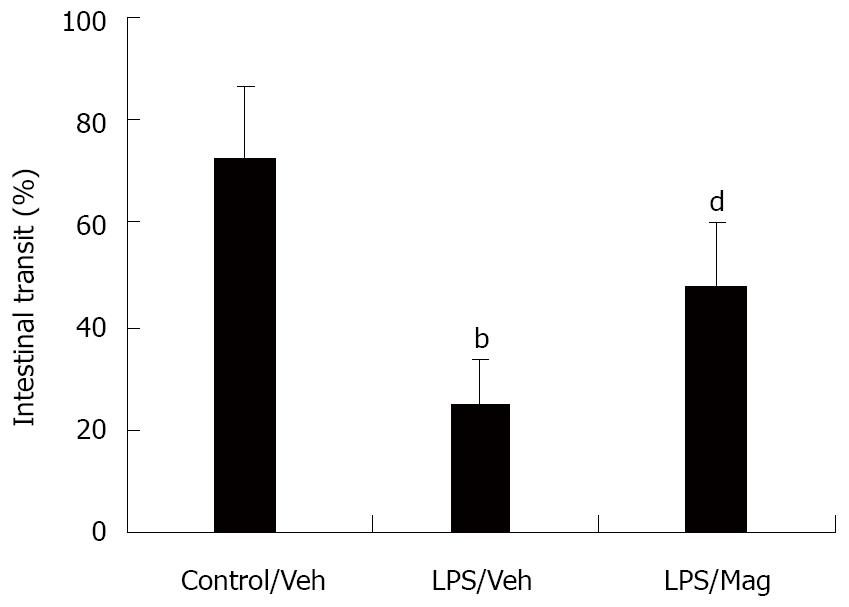
As shown in Figure 1, LPS significantly delayed small intestinal transit from 74% ± 14% in control rats to 25% ± 9% in LPS rats. Pretreatment with magnolol significantly increased the transit in LPS animals, although this increase did not return to the control distribution pattern.
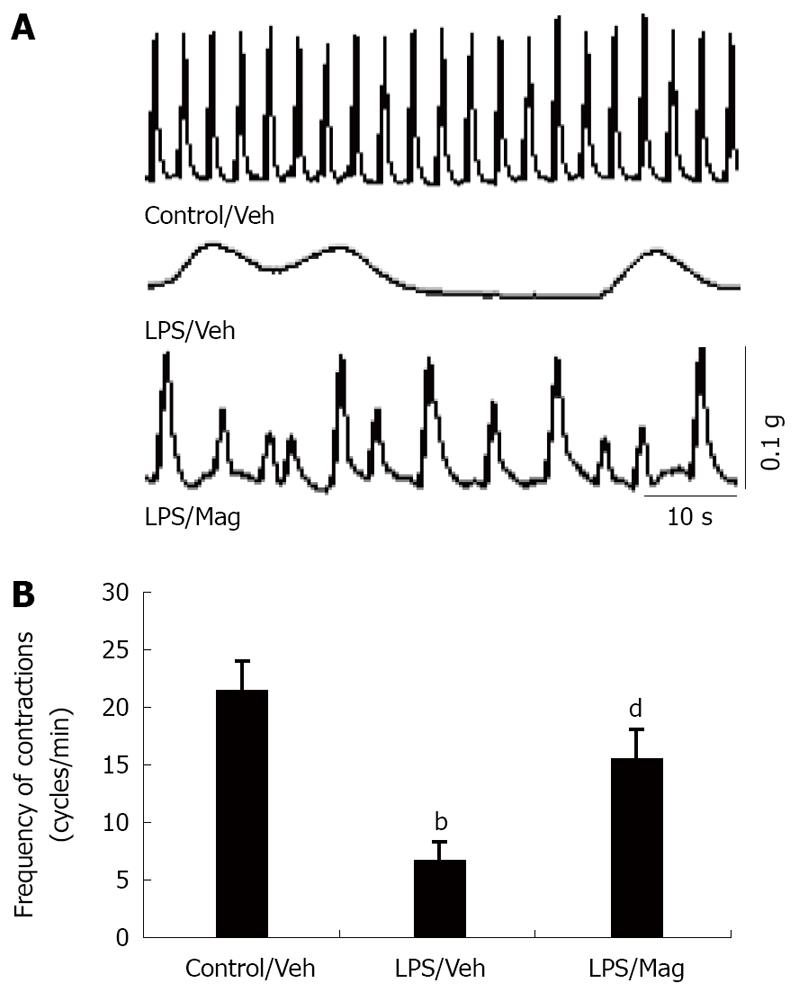
The second series of experiments was designed to determine the effect of intravenous magnolol pretreatment on the intestinal musculature by measuring in vitro ileal circular muscle contractility from septic animals after LPS injection. Figure 2A shows the typical spontaneous contractility of circular muscle strips from three different animals. Analysis of the frequency of spontaneous contraction showed that muscle contractility in LPS-treated intestines was significantly lower than that in control tissues. Pretreatment with magnolol partly restored the spontaneous contractile pattern (Figure 2B).
Next, we evaluated the contractile response of muscle strips to the muscarinic receptor agonist bethanechol (0.1-100 μmol/L) using isometric force measurements.
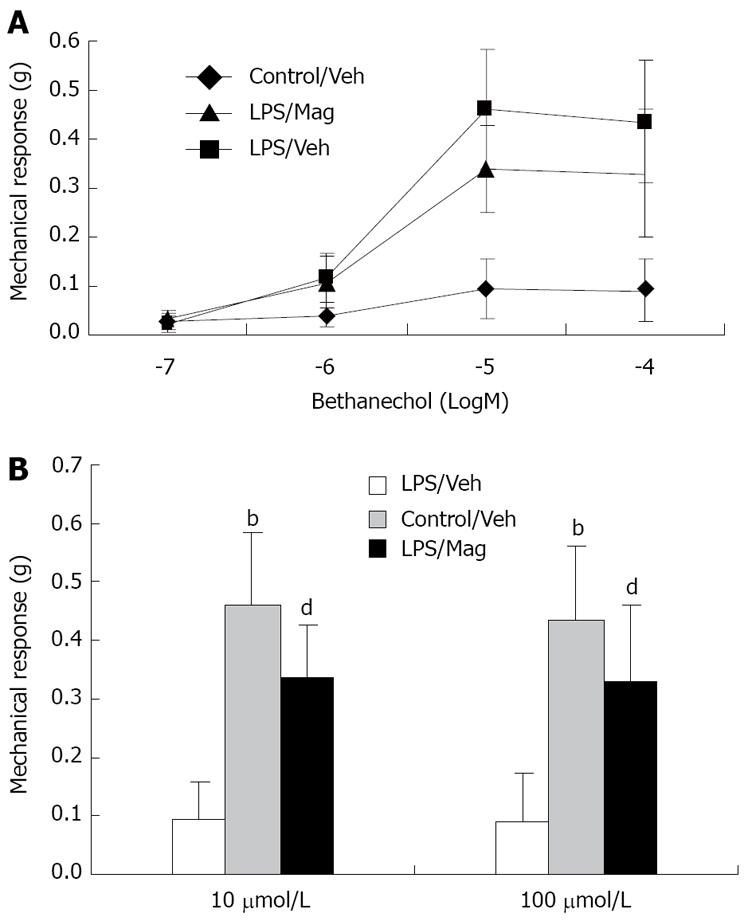
As shown in Figure 3A, ileal circular muscle strips from LPS-treated animals showed significant impairment in the dose-response curve of bethanechol-stimulated muscle contraction. Magnolol treatment partly prevented LPS-induced impairment of ileal circular smooth muscle contractility. Figure 3B shows that, compared with controls, LPS significantly suppressed bethanechol-induced circular muscle contractions at bethanechol concentrations of 10 and 100 μmol/L. Magnolol treatment significantly increased the mechanical response of ileal circular muscles in LPS-treated animals.
The effect of magnolol treatment on GI motility of control rats was also evaluated. Neither intestinal transit nor circular muscle strip contractility was altered by magnolol (data not shown).
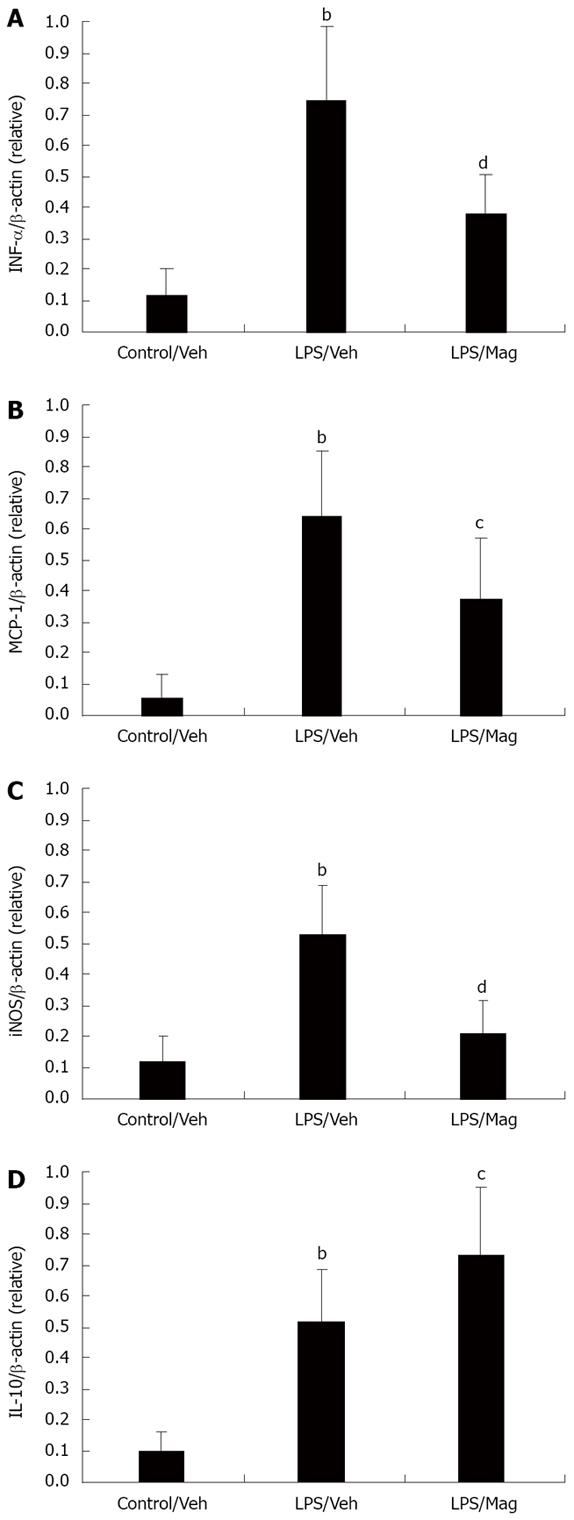
As shown in Figure 4, LPS induced a significant increase in TNF-α, IL-10 and MCP-1 mRNA levels in the ileum. Magnolol treatment significantly decreased LPS-induced TNF-α and MCP-1 mRNA expression. As for the anti-inflammatory mediator IL-10, magnolol significantly increased IL-10 mRNA expression in the ileum of LPS-treated animals.
iNOS has been shown to be the most important mediator of smooth muscle contraction during sepsis. Therefore, we also explored the effect of magnolol on iNOS mRNA expression in the ileum. Magnolol significantly suppressed LPS-induced iNOS mRNA expression.
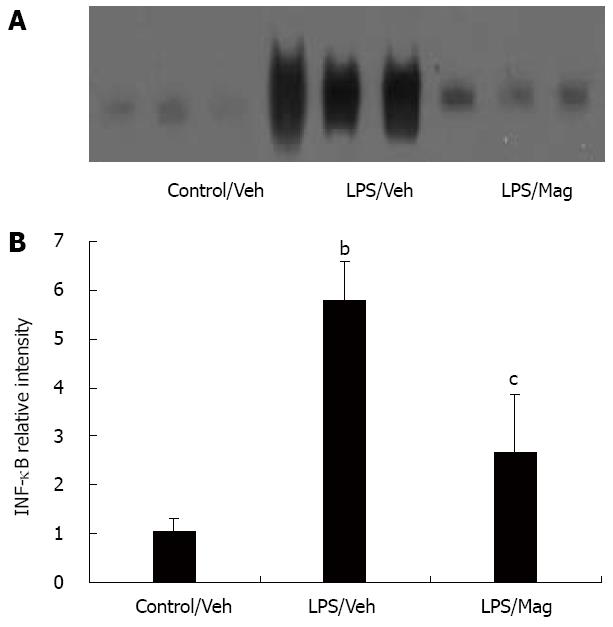
NF-κB comprises a family of transcription factors that act as regulators of pro-inflammatory mediators[16]. We hypothesized that magnolol could potentially produce the above beneficial effects through decreased expression of NF-κB. As shown in Figure 5, LPS significantly induced activated NF-κB above control levels, and as hypothesized, magnolol significantly suppressed this response.
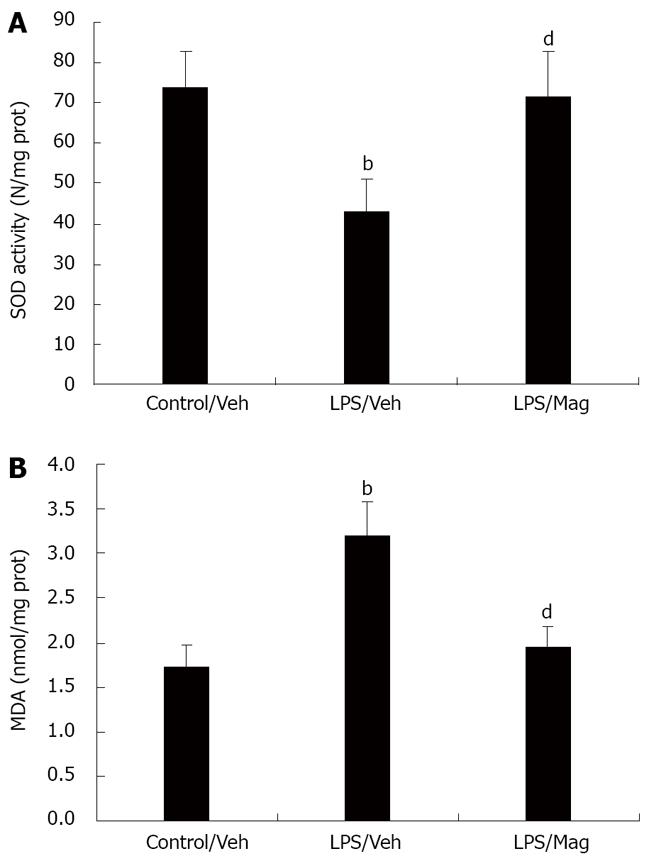
As shown in Figure 6, the MDA concentration in rat ileum, an index of lipid peroxidation, was significantly increased after LPS challenge compared with controls. Pretreatment with magnolol significantly decreased the MDA conce-ntration. SOD activity in intestinal tissue decreased markedly in LPS-treated animals. Magnolol pretreatment caused a significant increase in SOD activity in rat ileum.
This study demonstrated the ability of magnolol, an antioxidant isolated from a Chinese herb, to prevent intestinal dysmotility in LPS-induced septic rats. It also provided evidence that the potential mechanism of action of magnolol results from both attenuation of peroxidative damage and modulation of the inflammatory response during sepsis.
Sepsis-induced ileus after complicated abdominal surgery, hemorrhagic shock, trauma and burns still causes morbidity and mortality in critically ill patients. Accumulating evidence has indicated that overwhelming pro-inflammatory and oxidative stress responses combined with diminished anti-inflammatory pathways are responsible for GI dysmotility during sepsis[4-7]. Unfortunately, there is no accepted pharmacological prevention or management of sepsis-induced ileus at present. The present study demonstrated that magnolol can partly restore the delayed intestinal transit caused by LPS. Additionally, magnolol treatment can prevent LPS-induced impairment of ileal circular smooth muscle contractility.
Numerous studies have demonstrated that exaggerated production of oxygen-derived free radicals in the face of defective antioxidative protection occurs in animals and humans with sepsis[17-19]. This imbalance between pro- and anti-oxidants may produce oxidative stress, which ultimately leads to cellular injury and necrosis, via several mechanisms including lipid peroxidation, protein denaturation, and DNA damage. Magnolia officinalis has been used as a blood-quickening and stasis-dispelling agent in traditional Chinese medicine. Magnolol, a compound purified from the bark of Magnolia officinalis, has been shown to be 1000 times more potent than α-tocopherol in inhibiting lipid peroxidation in rat heart mitochondria[20], and 50 000 times more potent than glutathione, a well-known antioxidant. It can also exhibit free radical scavenging activity. Moreover, it has been reported to suppress superoxide anion production in myocardium exposed to ischemia and reperfusion[21]. In accordance with these studies, we found that magnolol significantly attenuated the intensity of lipid peroxidation and increased SOD activity in rat ileum during sepsis. The antioxidant properties of magnolol are proposed to underlie its beneficial effects during sepsis.
We considered that prevention of sepsis-induced intestinal dysmotility by magnolol was partly through interruption of the cycle of inflammatory events in the local intestine. To confirm this hypothesis, we performed semi-quantitative RT-PCR on ileal tissue for inflammatory cytokines TNF-α, MCP-1 and iNOS, which have been shown to participate in leukocyte recruitment and functional muscle impairment[4,5,22]. The anti-inflammatory mediator IL-10 was also evaluated in our experiment. We found that magnolol significantly suppressed the initial surge of TNF-α at the gene level and increased IL-10 expression in septic rat intestine. Pro-inflammatory cytokines, such as TNF-α, have been shown to be released early after an inflammatory stimulus[23]. The increase in pro-inflammatory cytokines is followed by an increase in anti-inflammatory cytokines, such as IL-10, which reflect the compensatory anti-inflammatory response syndrome[24]. It has been reported that IL-10 can inhibit cytokine production in monocytes by blocking LPS-induced NF-κB activation[25]. Additionally, IL-10 modulates the production of various chemokines (such as MCP-1) and prevents generation of NO by LPS-activated monocytes/macrophages[26-28].
MCP-1 is a potent chemoattractant that is capable of promoting monocyte recruitment into an inflammatory site, as well as activating monocytes and macrophages[29,30]. It has previously been shown that regulation of leukocyte recruitment and subsequent intestinal smooth muscle dysmotility during endotoxemia is mediated through MCP-1, and that a major source of MCP-1 is the dense network of resident muscularis macrophages[13]. In this study, we used MCP-1 mRNA as our marker of chemokine activity. As mentioned above, magnolol significantly reduced intestinal MCP-1 mRNA expression during LPS-induced sepsis.
NO is known to be the main inhibitory neurotransmitter of the GI tract, caused by the activity of the constitutive isoform of neural NO synthase (cNOS) within the enteric nervous system[31]. Besides, NO is produced at almost all sites of inflammation by leukocytes through the activity of iNOS[32]. Evidence indicates that NO from iNOS plays a pivotal role in mediating LPS-induced suppression of intestinal smooth muscle activity[4], and that up-regulation of iNOS activity is mediated by TNF-α and IL-1[5]. Additionally, NO and superoxide anions can join to form the toxic metabolite ONOO-[33,34], which is also involved in the pathogenesis of sepsis-induced motility disturbances[7]. Magnolol has been reported to suppress the overproduction of NO and TNF-α in LPS-activated macrophages[35]. The results obtained in the present study provide support for this view. Pretreatment with magnolol significantly decreased iNOS mRNA expression in the intestine of the LPS-treated animals.
NF-κB is an inducible nuclear transcription factor that plays a central role in regulating the transcription of many pro-inflammatory cytokines[16], including TNF-α and IL-1β. Furthermore, intricate negative and positive feedback loops exist within NF-κB activation and cytokine expression. Pro-inflammatory cytokines activate NF-κB, but IL-10 deactivates NF-κB[36]. In the present study, we found that magnolol significantly suppressed NF-κB activation in the intestine of septic rats, which suggests that magnolol modulates inflammatory cytokines may be through intervention in the NF-κB signal transduction system. In addition, magnolol might also inhibit NF-κB activation through increasing IL-10 gene expression.
During sepsis, oxidative stress causes direct damage to cells and tissues and is involved with inflammatory cytokine production[17]. Suppression of cytokines by antioxidants has been demonstrated in previous studies. N-acetyl-cysteine has been shown to prevent the priming of increased expression of TNF-α mRNA after LPS[37]. Also, it has been reported that the free-radical-trapping compound phenyl N-tert-butylnitrone administered in LPS-induced sepsis promotes enhanced production of endogenous IL-10[38]. Additionally, the involvement of oxidative stress or oxygen free radicals in NF-κB activation has been suggested[39]. Therefore, we assume that magnolol modulation of cytokine synthesis may be related to its antioxidant properties. This is in agreement with previous studies that have shown that gut injury is partly prevented by antioxidants[40]. However, this has not been proven experimentally.
Although the findings of the present study predict a role for magnolol in a clinical setting, several problems should be mentioned. We did not use the cecal ligation and puncture (CLP) sepsis model in our study, which appears to be a reliable and clinically relevant animal model of the human septic condition, because abdominal surgery can also initiate an inflammatory cascade and ultimately lead to impairment of intestinal smooth muscle activity. More intricate pathophysiological mechanisms may be involved in the development of gut dysmotility in the CLP sepsis model[41]. Additionally, Zhang et al[42] previously reported that, in vitro, magnolol exerted an inhibitory effect on isolated ileum of guinea pigs. However, we found in our study that in vivo, magnolol treatment could prevent LPS-induced suppression of intestinal motility but had no effect on control animals. These discrepancies suggest that the pharmacological properties of magnolol on GI motility might change when it is administered at different doses or via different routes. At the dose and route that we used in our study, the antioxidant effect of magnolol could be the important mechanism through which it ameliorates the severity of sepsis. Under other pathophysiological conditions, whether magnolol could exert a similar effect is still not known. Other well-designed experiments are needed to further determine the clinical usefulness and safety of magnolol.
In conclusion, the data presented in this study suggest a protective role of magnolol in preventing sepsis-induced suppression of intestinal motility. The potential mechanism of this beneficial effect of magnolol appears to be modulation of the self-amplified inflammatory events and block of oxidative stress in the intestine.
During sepsis, gastrointestinal (GI) dysmotility occurs frequently. Accumulating evidence has indicated that overwhelming pro-inflammatory and oxidative stress responses combined with diminished anti-inflammatory pathways are responsible. Recently, magnolol, an antioxidant isolated from a traditional Chinese herb, has been showed to attenuate peroxidative damage and to improve survival of rats with sepsis. It can also suppress the TNF-α level and preserve IL-10 production in hemorrhagic shock in rats. Thus, the authors presumed that through modulation of inflammatory cytokines during sepsis, magnolol might be helpful for treatment of sepsis-induced ileus.
Sepsis-induced GI dysmotility is a major problem in critically ill patients. The pharmacological intervention is difficult for the clinician to handle. In addition, there is a lack of controlled studies on which to build an evidence-based treatment concept for critically ill patients.
Currently, there is no accepted pharmacologic prevention or management of sepsis-induced GI dysmotility. Therefore, management remains largely supportive. Insights gained in this preliminary study might be helpful in producing an effective pharmacological intervention strategy.
This study provides the evidence that pretreatment with magnolol could attenuate sepsis-induced GI dysmotility. The potential mechanism of this benefit of magnolol appears to be modulation of the self-amplified inflammatory events and block of oxidative stress in the intestine.
Sepsis is defined as infection plus systemic manifestations of infection. Cytokines: non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, which act as intercellular mediators. Magnolol (5,5’-di-2-propenyl-1,1’-biphenyl-2,2’-diol), a principal constituent isolated from a traditional Chinese herb. Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria, act as endotoxins and elicit strong immune responses in animals.
This preliminary study provides us with a new insight into management of sepsis-induced GI dysmotility. However, the pharmacological properties of magnolol may change when it is administered at different doses or via different routes. Other well-designed experiments are needed to further determine its clinical utility and safety.
Peer reviewer: Susumu Ohwada, Associate Professor, Department of Surgery, Gunma University Graduate School of Medicine, 3-39-15 Shoma-Machi, Maebashi 371-8511, Japan
S- Editor Cheng JX L- Editor Cant MR E- Editor Yin DH
| 1. | Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. |
| 2. | Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196-208. |
| 3. | MacFie J, O'Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223-228. |
| 4. | Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol. 1999;277:G478-G486. |
| 5. | Lodato RF, Khan AR, Zembowicz MJ, Weisbrodt NW, Pressley TA, Li YF, Lodato JA, Zembowicz A, Moody FG. Roles of IL-1 and TNF in the decreased ileal muscle contractility induced by lipopolysaccharide. Am J Physiol. 1999;276:G1356-G1362. |
| 6. | Torihashi S, Ozaki H, Hori M, Kita M, Ohota S, Karaki H. Resident macrophages activated by lipopolysaccharide suppress muscle tension and initiate inflammatory response in the gastrointestinal muscle layer. Histochem Cell Biol. 2000;113:73-80. |
| 7. | de Winter BY, van Nassauw L, de Man JG, de Jonge F, Bredenoord AJ, Seerden TC, Herman AG, Timmermans JP, Pelckmans PA. Role of oxidative stress in the pathogenesis of septic ileus in mice. Neurogastroenterol Motil. 2005;17:251-261. |
| 8. | Wang HL, Bai H, Wang XQ, Li Y. Experimental study of effect of Magnolia officinalis cotex on improving rat gastrointestinal motility. Shiyong Yaowu Yu Linchuang. 2007;10:65-66. |
| 9. | Ci XL, Wang BE, Guo CY, Chen L. Experimental study of effect of Magnolia officinalis on improving the electricity activity of gastrointestinal smooth muscle in septic rats. Zhongguo Zhongyiyao Keji. 1999;6:154-156. |
| 10. | Kong CW, Tsai K, Chin JH, Chan WL, Hong CY. Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock. 2000;13:24-28. |
| 11. | Shih HC, Wei YH, Lee CH. Magnolol alters cytokine response after hemorrhagic shock and increases survival in subsequent intraabdominal sepsis in rats. Shock. 2003;20:264-268. |
| 12. | Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol. 1997;273:G727-G734. |
| 13. | Wirthlin DJ, Cullen JJ, Spates ST, Conklin JL, Murray J, Caropreso DK, Ephgrave KS. Gastrointestinal transit during endotoxemia: the role of nitric oxide. J Surg Res. 1996;60:307-311. |
| 14. | Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003;9:1567-1570. |
| 15. | Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor kB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346-3492. |
| 16. | Abraham E. NF-kappaB activation. Crit Care Med. 2000;28:N100-N104. |
| 17. | Goode HF, Webster NR. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21:1770-1776. |
| 18. | Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135-159. |
| 19. | Albuszies G, Bruckner UB. Antioxidant therapy in sepsis. Intensive Care Med. 2003;29:1632-1636. |
| 20. | Lo YC, Teng CM, Chen CF, Chen CC, Hong CY. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol. 1994;47:549-553. |
| 21. | Lee YM, Hsiao G, Chen HR, Chen YC, Sheu JR, Yen MH. Magnolol reduces myocardial ischemia/reperfusion injury via neutrophil inhibition in rats. Eur J Pharmacol. 2001;422:159-167. |
| 22. | Turler A, Schwarz NT, Turler E, Kalff JC, Bauer AJ. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G145-G155. |
| 23. | Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino MA Jr, Cerami A, Shires GT, Lowry SF. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988;166:147-153. |
| 24. | Molloy RG, Mannick JA, Rodrick ML. Cytokines, sepsis and immunomodulation. Br J Surg. 1993;80:289-297. |
| 25. | Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558-9563. |
| 26. | de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209-1220. |
| 27. | Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815-3822. |
| 28. | Ikeda T, Sato K, Kuwada N, Matsumura T, Yamashita T, Kimura F, Hatake K, Ikeda K, Motoyoshi K. Interleukin-10 differently regulates monocyte chemoattractant protein-1 gene expression depending on the environment in a human monoblastic cell line, UG3. J Leukoc Biol. 2002;72:1198-1205. |
| 29. | Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990;11:97-101. |
| 30. | Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769-5776. |
| 31. | Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991;444:743-761. |
| 33. | Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease--radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997-2015. |
| 34. | Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79-88. |
| 35. | Son HJ, Lee HJ, Yun-Choi HS, Ryu JH. Inhibitors of nitric oxide synthesis and TNF-alpha expression from Magnolia obovata in activated macrophages. Planta Med. 2000;66:469-471. |
| 36. | Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3-9. |
| 37. | Fan J, Kapus A, Li YH, Rizoli S, Marshall JC, Rotstein OD. Priming for enhanced alveolar fibrin deposition after hemorrhagic shock: role of tumor necrosis factor. Am J Respir Cell Mol Biol. 2000;22:412-421. |
| 38. | Kotake Y, Sang H, Tabatabaie T, Wallis GL, Moore DR, Stewart CA. Interleukin-10 overexpression mediates phenyl-N-tert-butyl nitrone protection from endotoxemia. Shock. 2002;17:210-216. |
| 39. | Schreck R, Baeuerle PA. Assessing oxygen radicals as mediators in activation of inducible eukaryotic transcription factor NF-kappa B. Methods Enzymol. 1994;234:151-163. |
| 40. | Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117-134. |
| 41. | Overhaus M, Tögel S, Pezzone MA, Bauer AJ. Mechanisms of polymicrobial sepsis-induced ileus. Am J Physiol Gastrointest Liver Physiol. 2004;287:G685-G694. |
| 42. | Zhang WW, Li Y, Wang XQ, Tian F, Cao H, Wang MW, Sun QS. Effects of magnolol and honokiol derived from traditional Chinese herbal remedies on gastrointestinal movement. World J Gastroenterol. 2005;11:4414-4418. |