Published online Jan 28, 2008. doi: 10.3748/wjg.14.601
Revised: November 16, 2007
Published online: January 28, 2008
AIM: To determine the clinical, radiographic and pathologic characteristics, diagnostic and treatment modalities in patients with autoimmune pancreatitis (AIP).
METHODS: In this retrospective study, the data of patients with diagnosed chronic pancreatitis (CP) between 1995 and 2006 in Chinese PLA General Hospital were included to screen for the cases with AIP, according to the following diagnostic criteria: (1) diagnostic histopathologic features, and abound IgG4-positive plasma cells on pancreatic tissues; (2) characteristic imaging on computed tomography and pancreatography, together with increased serum IgG, γ-globulin levels or presence of autoantibodies; (3) response to steroid therapy. The clinical, radiographic and pathologic characteristics, diagnostic and treatment modalities, and outcome of AIP cases were reviewed.
RESULTS: Twenty-five (22 male, 3 female; mean age 54 years, 36-76 years) out of 510 CP patients were diagnosed as AIP, which accounted for 49% (21/43) of CP population undergoing surgical treatment in the same period. The main clinical manifestations included intermittent or progressive jaundice in 18 cases (72%), abdominal pain in 11 (44%), weight loss in 10 (40%), and 3 cases had no symptoms. The imaging features consisted of pancreatic enlargement, especially in the head of pancreas (18 cases), strictures of main pancreatic duct and intrapancreatic bile duct. Massive lymphocytes and plasma cells infiltration in pancreatic tissues were showed on pathology, as well as parenchymal fibrosis. Twenty-three patients were misdiagnosed as pancreaticobiliary malignancy, and 21 patients underwent exploratory laparotomy, the remaining 4 patients dramatically responded to steroid therapy. No pancreatic cancer occurred during a mean 46-mo follow-up period.
CONCLUSION: AIP patients always are subjected to mistaken diagnosis of pancreatic cancer and an unnecessary surgical exploration, due to its similarity in clinical features with pancreatic cancer. The differential diagnosis with high index of suspicion of AIP would improve the diagnostic accuracy for AIP.
- Citation: Song Y, Liu QD, Zhou NX, Zhang WZ, Wang DJ. Diagnosis and management of autoimmune pancreatitis: Experience from China. World J Gastroenterol 2008; 14(4): 601-606
- URL: https://www.wjgnet.com/1007-9327/full/v14/i4/601.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.601
Autoimmune pancreatitis (AIP) is a unique chronic inflammation of the pancreas in which autoimmune mechanisms are involved in the pathogenesis[1–3]. Since Sarles et al first described AIP case in 1961[4], increasing numbers of AIP patients have been diagnosed worldwide[5–9]. Although AIP has distinct characteristics at clinical, radiologic, histologic, and serologic findings[1–38], due to the extreme similarity in clinical manifestations of pancreaticobiliary malignancies, the benign condition of AIP has frequently been diagnosed or suspected as pancreatic cancer, especially when clinicians have no awareness of AIP, which consequently impose the patients an unnecessary pancreatic resection[10–12].
The diagnosis of AIP is mainly based on the diagnostic criteria proposed by the Japan Pancreas Society (JPS) in 2002[13]. However, these criteria preclude many problematic cases with clinical findings compatible with AIP, as well as with excellent response to steroids, as a result of failure to fulfill the JPS criteria. Recently, some revised or new diagnostic criteria have been proposed[13–15], including the Mayo Clinic Criteria.
In this study, we reviewed 25 AIP patients from a single institution in China, and analyzed the clinical, radiographic and pathologic characteristics, diagnostic and treatment modalities, outcome, especially the encountered lessons, in order to improve the diagnostic accuracy for patients with AIP.
The preliminary screening for AIP patients was carried out among 510 patients who were diagnosed as chronic pancreatitis (CP) between January 1995 and June 2006 in Chinese PLA General Hospital. Those who fit the etiology and diagnostic pictures of alcoholic, gallstone, or other recognized causes of classical CP were excluded initially[16], then the diagnosis of AIP was made in patients with ≥ 1 of these criteria among the remaining patients: (1) diagnostic histopathologic features, and abound IgG4-positive plasma cells on pancreatic tissues; (2) characteristic imaging on computed tomography and pancreatography, together with increased serum IgG, γ-globulin levels or presence of autoantibodies; (3) response to steroid therapy. Above diagnostic criteria derived from the combination of HISORt (histology, imaging, serology, other organ involvement, and its response to steroid therapy) criteria of Mayo Clinic[15] and other diagnostic criteria[11].
Finally, twenty-five patients met the above criteria for AIP, accounting for 4.9% of CP patients in the same time period.
The medical records of the 25 patients (22 males, 3 females; mean age 54 years, range 36-76 years) were reviewed. The mean course of onset of AIP is 2-mo (1-14 mo).
Ultrasonography and computerized tomography (CT) scanning were performed for all 25 patients, and endoscopic retrograde cholangiopancreatography (ERCP) was performed in 11 patients, and magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) were done in 7 patients.
Ultrasound-assisted biopsy specimens of pancreatic lesions were obtained in 6 patients prior to surgery; and intraoperative resected or biopsy specimens of pancreatic lesions were obtained in 21 patients with AIP (2 had both pre- and intra-operative specimens). The specimens were fixed in 10% formaldehyde and embedded in paraffin. The pathologic diagnosis of AIP was made by the hematoxylin and eosin (HE)-stained sections and immunohistochemical staining using CD4, CD8, and IgG4 antibodies.
The main clinical manifestations included intermittent or progressive jaundice in 18 patients (72%), mild epigastric pain or discomfort in 11 (44%), weight loss in 10 (40%), low-grade fever and chronic diarrhea in 2 each. Three patients had no symptoms. Twenty-four percent (6/25) of the AIP patients had a history of autoimmune disease (ulcerative colitis, n = 2; asthma, n = 1; Sjögren’s syndrome, n = 1; systemic lupus erythematosus, n = 1; retroperitoneal fibrosis and nephrydrosis, n = 1); other comorbidities included diabetes mellitus (n = 5), chronic acalculous cholecystitis (n = 2), and suspected diagnosis of primary sclerosing cholangitis (n = 2). None of these patients had a history of excess alcohol consumption, or gallstones.
Twenty patients (80%) had obstructive jaundice and abnormal serum levels of aminotransferases (ALT and AST) and biliary enzymes (alkaline phosphatase, γ-glutamyltransferase). Hyperglycemia was found in 7 patients (28%), and abnormality of serum CA19-9 level (> 37 U/L) was found in 12 patients (12/19, 63%), among them, the CA19-9 level above 400 U/L in 4 patients. Abnormally elevated levels of serum γ-globulin and IgG were seen in 4 (4/5, 80%). The autoantibodies were determined in 5 patients, only rheumatoid factor, and anti-SSA/-SSB autoantibodies presented in 1 patient each. Mild elevated level of anti-mitochondrial antibody was found in 2. Other antibodies such as antinuclear antibody, antismooth muscle antibody were all negative. No hyperamylasemia was found in all patients.
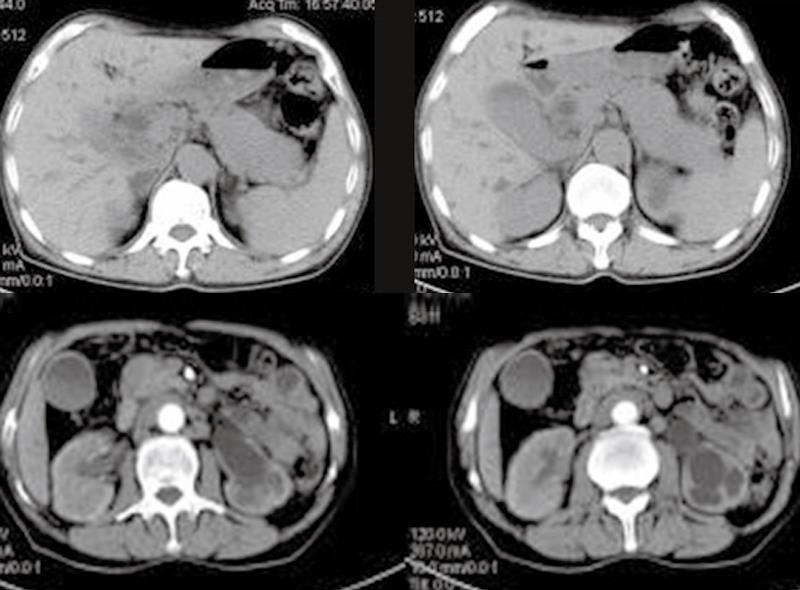
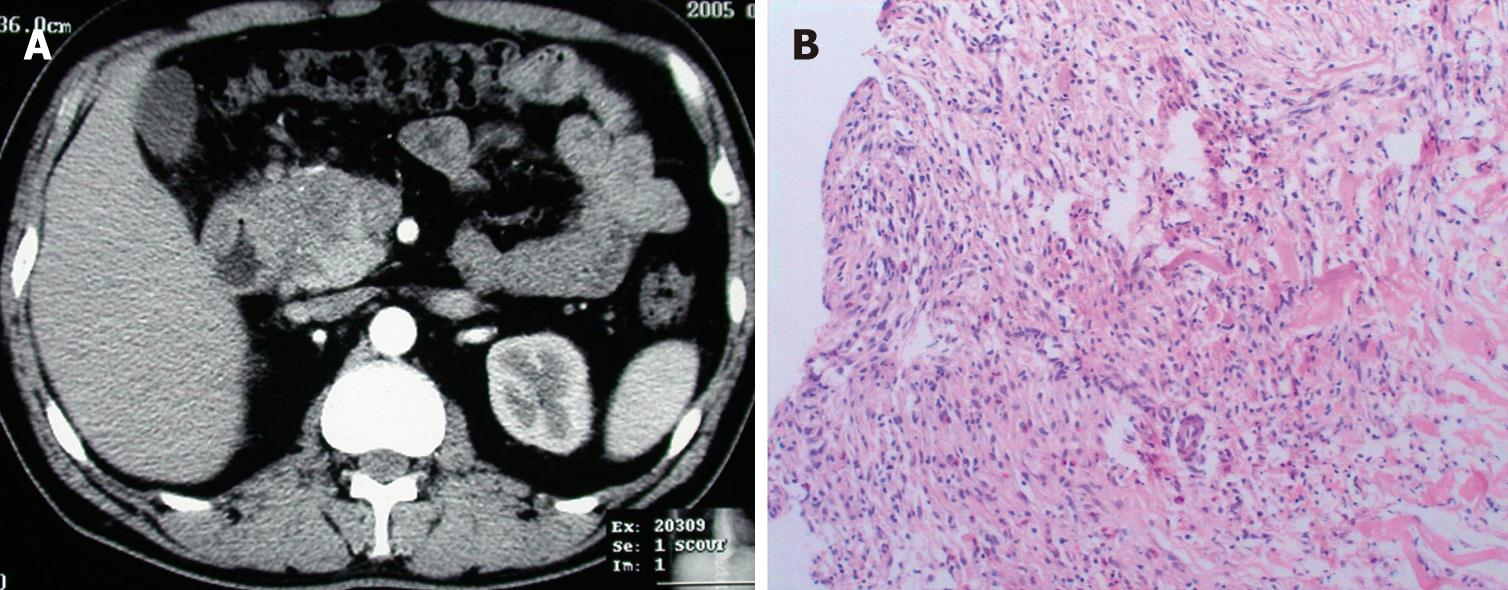
The results of ultrasonography, CT, and MRI imaging demonstrated that all patients had abnormal alterations in pancreas, massive pancreatic enlargement (sausage-shaped, Figure 1) in 7 patients, and swelling of pancreatic head in 18 patients (Figure 2); the maximal diameter of pancreatic head > 5 cm in 6, and dilation of biliary trees in 18, and thickening of extra-hepatic biliary (Figure 3) or gallbladder walls in 8, retroperitoneal fibrosis and nephrydrosis in 1 (Figure 1).
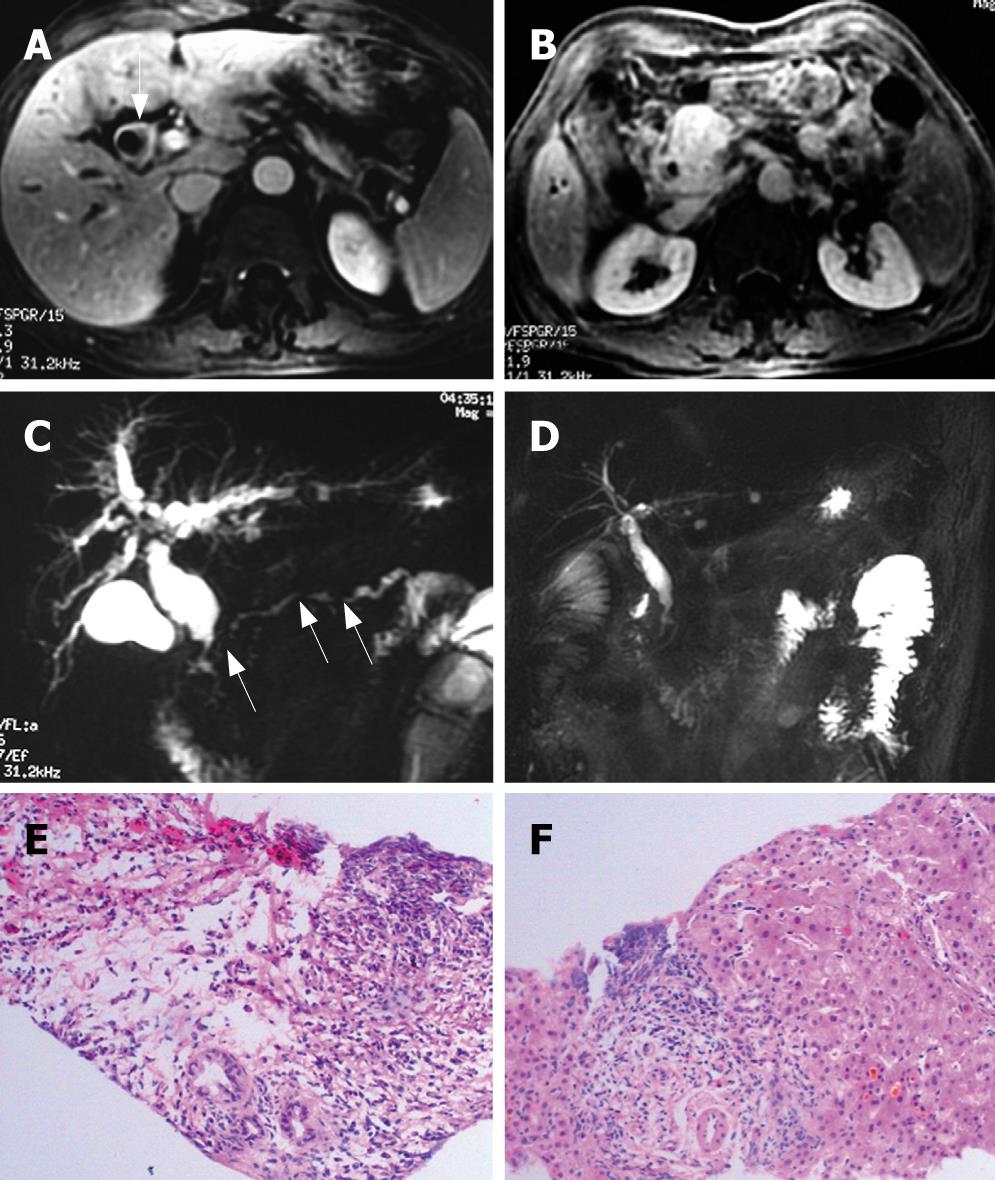
Ultrasonography revealed a suspected pancreatic cancer in 18 cases (72%), and biliary cancer in 4 cases (16%), and CP was considered in 3. CT scanning showed pancreatic cancer in 15 (60%), and cholangiocarcinoma in 8 (32%), and CP was suspected in 2, for whom, pancreaticobiliary malignancy was not excluded. ERCP and MRI/MRCP were detected in 11 cases and 7 cases, respectively, all revealed a stricture of intra-pancreatic common bile duct (CBD) with dilation of upstream biliary trees, focal stricture of hilar duct in 5; multiple stricture of main pancreatic duct (MPD) in 8, and segmental strictures of MPD with minimal upstream dilation in 6 (Figure 3), near-normal configuration of MPD in 2, and poor visualization of MPD in 2.
Based on the clinical and imaging features, a diagnosis or suspected diagnosis of pancreatic cancer and biliary cancer were made in 15 and 8 cases, respectively, and with a 92 percent of misdiagnosing rate at admission.
During surgery, the pancreas was “rock-hard” or firm at palpation, as well as peripancreatic inflammatory adhesion, for whom pancreatic cancer was not ruled out. No calcification, mucoprotein plugs, or pseudocysts was exhibited.
Histological examinations show a significant presence of lymphocytes, plasma cell infiltration surrounding medium and large interlobular ducts, obliterative phlebitis, and scattered eosinophils, as well as acinar atrophy and dense fibrosis in all specimens (Figures 2 and 3). In specimens of 8 cases, dense fibrosis completely replaced the acinar cells and islets, with loss of figuration of pancreatic tissue. Immunohistochemical staining revealed a predominance of CD8+ and CD4+ T lymphocytes, with few B lymphocytes, as well as abundant IgG4-positive plasma cells.
Above-mentioned pathologic features are also present in biliary duct, gallbladder, and liver (Figure 3). In the liver specimens, cholestasis, diffusely cloudy swelling of hepatocytes, and prominent lymphoplasmacytic infiltration are detected, especially at the portal area.
Twenty-one patients with AIP underwent 22 sessions of exploratory laparotomy, accounting for 49% (21/43) of the patients with diagnosed CP who undergoing laparotomy: 4 cases underwent pancreaticoduodenectomy (PD) owing to suspicion of pancreatic cancer; 14 cases underwent choledochojejunostomy, among them, 1 case performed 2 sessions of laparotomy (the first laparotomy at a local hospital revealed a firm palpation of pancreatic head and portal vein encasement, and a presumptive diagnosis of advanced pancreatic cancer was made, together with a biliary external drainage; 4 mo later choledochojejunostomy was performed in our hospital after pathologic diagnosis of AIP); for the 3 asymptomic patients, 1 with preoperative diagnosis of AIP insisted on surgery and thus radiofrequency ablation for the enlarged pancreatic head was performed, another 2 only performed intraoperative biopsy of pancreatic tissues. Nineteen of 20 patients with obstructive jaundice underwent surgery, because of the mistaken diagnosis of pancreaticobiliary cancer. Four patients with pathologic diagnosis of AIP through ultrasound-guided percutaneous pancreatic biopsy did not undergo laparotomy.
Four patients take oral prednisolone, and repeated CT and/or MRI revealed improvement of pancreatic imaging 1 mo after steroid therapy (Figure 3), the clinical manifestation and serum levels of γ-globulin, IgG, and CA19-9 restored normally. Furthermore, the effective response to steroid therapy confirmed the diagnosis of AIP for the suspected cases. After steroid therapy, the abnormality of biliary duct and pancreatic duct normalized, and the nephrydrosis improved significantly, but not the retroperitoneal fibrosis.
All the patients with AIP were followed-up, with a mean period of 46 mo (12-142 mo), and no pancreaticobiliary cancers were progressed, nor the symptoms of jaundice, abdominal pain, or acute episode of acute pancreatitis.
Autoimmune pancreatitis (AIP) is a distinct subtype of CP. AIP occurs in the absence of gallstones, pancreas divisum, alcohol abuse, or other factors commonly associated with CP. AIP is characterized histologically by a diffuse lymphoplasmacytic infiltration and dense fibrosis. AIP was first described in 1961 by Sarles et al[4], who reported 2 patients with hypergammaglobulinemia. The concept of AIP was first proposed by Yoshida et al in 1995[1]. Hereafter, the concept of AIP has been widely accepted, and its clinical, histologic, radiographic, and serologic features have been characterized[5–12]. The cellular mechanisms and genetic factors contributing to AIP are also investigated[17–19].
The following characteristics of AIP have been noted[5–12]: (1) predominance of elder male, with mean age of 55-63 years; (2) nonspecific abdominal pain, painless jaundice (> 60%), and, rarely, acute attack of pancreatitis; (3) dense fibrosis with prominent lymphoplasmacytic infiltration in pancreatic lesions; (4) diffuse or focal irregular narrowing of the MPD on ERCP, together with stricture of intrapancreatic common bile duct and dilation of the bile duct upstream; (5) diffuse or focal enlargement of the pancreas; (6) increased serum γ-globulin or IgG or IG4 levels; (7) the presence of autoantibodies; (8) frequent presence of cholestatic liver dysfunction, hyperbilirubinemia, and elevated CA19-9 level; (9) usually absence of pancreatic calcification and cysts; (10) extra-pancreatic organs involvement in autoimmune diseases, such as ulcerative colitis, Sjögren’s syndrome, sclerosing cholangitis; and (11) effectiveness of steroid therapy. Sometimes, AIP patients presented with nephrydrosis or upper gastrointestinal bleeding[20].
Nevertheless, the clinical manifestations of AIP are nonspecific, which makes it difficult to differentiate AIP from pancreaticobiliary cancer. Therefore, a significant number of patients with AIP receive operative treatment for an inflammatory mass suspicious of pancreatic cancer. The incidence of AIP ranges 1.86%-6.6% of CP[21], however, the AIP patients undergoing PD due to a clinical suspicion of a malignancy ranges 21%-28% of CP patients receiving surgery[102223], the AIP lesions sometimes have been identified as unresectable tumor because of vessel infiltration.
In China, few cases with AIP have been reported[9]. In our experience, the AIP patients accounted for 5% of CP population; however, 84% (23/25) of the AIP patients was diagnosed or suspected of pancreaticobiliary malignancy, which resulted in 22 episodes of laparotomy for 21 AIP cases, accounting for 49% (21/43) of CP patients receiving surgical treatment.
The pathological features of lymphoplasmacytic infiltration and fibrosis, acinar atrophy are often used as the gold standard for the diagnosis of AIP[1124–26]. However, the diagnosis made on pancreatic biopsy specimens may be inconclusive (false negative) or suggestive, because of small sample size and sampling error[2627]. For those with suspected pancreatic cancer, the role of needle or core biopsy specimen is primarily to exclude malignancy[24]. Although studies have revealed that IgG4-positive plasma cells may be a useful marker for the diagnosis of AIP[28–32], whether IgG4-positive staining can diagnose AIP with certainty needs further investigation[30–33].
Abdominal CT findings include a focally or diffusely pancreatic involvement, especially in the head of pancreas, with low-attenuation or an isoattenuation. Thus, the differential diagnosis of focal AIP from pancreatic cancer is difficult on CT findings only. On CT and MRI, the diffusely enlarged pancreas shows a sausage-like appearance, as well as a peripheral rim of the hypointense “halo”. The hallmark findings on ERCP are focal, segmental or diffuse irregular narrowing of the MPD, the upstream duct is minimally dilated. Occasionally, a double duct sign (concomitant presence of distal CBD and distal pancreatic duct stricture, suggesting a tumor in the head of pancreas) may be present. Endoscopic ultrasound in the diagnosis of AIP has not been well characterized; however, endoscopic ultrasound guided fine needle aspiration biopsy is beneficial to establish a diagnosis of AIP and exclude cancer[34].
MRCP poorly shows pancreatic duct strictures[35], but it can well demonstrate stricture of the intrapancreatic bile duct, and dilatation of the upper biliary tract. Sclerosing changes of the extrapancreatic bile ducts similar to PSC are sometimes observed. Steroid therapy is effective for changes of the biliary and pancreatic ducts. In view of its above-mentioned advantages and its noninvasiveness, MRCP can partially replace the role of ERCP in diagnosing of AIP[9].
AIP patients frequently exhibit positive serologic antibodies, and elevated levels of γ-globulin or IgG. It has reported that elevated levels of the IgG4 were a sensitive and specific marker of AIP[3637]; however, elevated level of IgG4 seems nonspecific for AIP[1338] and its role requires further study. In China, the determination of serum IgG4 level is still unavailable for most of hospitals, so we did not use it in our diagnostic criteria.
Current diagnostic criteria include the JPS criteria[1339], the Kim criteria[14], the HISORt criteria of Mayo Clinic[15], and the Italian criteria[13]. This absence of integrated criteria makes it difficult to compare studies from different centers. The problem of JPS criteria is that the cases showing an excellent response to steroids may yet fail to fulfill the JPS criteria[133940]. Thus other 3 criteria include the criterion “response to steroid therapy”[813–15]. The dramatic effectiveness of steroid for the suspected AIP cases strongly supports the diagnosis, thus avoiding unnecessary surgery.
Steroid therapy is effective for AIP. The initial dose of prednisone should be 30-40 mg daily for a week, followed by a taper of the daily dose by 2.5-5 mg per week. The response to steroids is often dramatic[13–1539]. During the first 2-4 wk of therapy, CT or MRI/MRCP should be used to monitor the response. Laboratory, and functional abnormalities may also show improvement with steroid therapy[13–153941]. A poor response to 1-2 wk of steroid therapy should raise the question of pancreatic cancer or other forms of CP. Surgery is preserved for those in whom the pancreaticobiliary malignancy could not be excluded or who complicated with infection of biliary tract.
In summary, the establishment of diagnosis of AIP should rely on combinations of the clinical, radiographic, histologic and serologic evidence, and a response to steroids. AIP is frequently misdiagnosed as pancreatic cancer, because of the clinical similarity with cancer. In order to improve the diagnostic accuracy for AIP, the clinicians must have a high index of suspicion of AIP and be conversant with its manifestations.
Autoimmune pancreatitis (AIP) is a unique chronic inflammation of the pancreas. AIP has distinct characteristics at clinical, radiologic, histologic, and serologic findings. However, due to the extreme similarity in clinical manifestations of pancreaticobiliary tumors, the patients with AIP frequently were misdiagnosed as pancreatic cancer, and were imposed an unnecessary pancreatic resection.
The establishment of reasonable diagnostic criteria compatible with all AIP patients is the major area of research, because the criteria can spare the AIP patients unnecessary pancreatic resection.
Although presence of established diagnostic criteria, that clinicians have the knowledge of AIP and with a high index of suspicion is the prerequisite for accurate diagnosis of AIP. In this retrospective study, we demonstrated that there is a terrible frequency of misdiagnosis and unnecessary surgery in AIP patients if the clinicians have no awareness of AIP.
The diagnostic accuracy for AIP will be improved, if the clinicians have a high index of suspicion of AIP and are conversant with its manifestations. The accurate diagnosis is mainly based on clinical, radiologic and histologic findings, sometimes together with excellent response to steroid therapy.
AIP is a type of chronic pancreatitis characterized by an autoimmune inflammatory process in which prominent lymphoplasmacytic infiltration and fibrosis of the pancreas.
This is the first English literature reporting AIP patients from China, most of the AIP patients in China were misdiagnosed and experienced subjected to unnecessary surgery, as their Western counterparts before the entity of AIP was not recognized widely. The diagnostic criteria used in this study are practical for AIP patients from developing countries, where the determination of serum IgG4 is unavailable.
| 1. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [Cited in This Article: ] |
| 2. | Ito T, Nakano I, Koyanagi S, Miyahara T, Migita Y, Ogoshi K, Sakai H, Matsunaga S, Yasuda O, Sumii T. Autoimmune pancreatitis as a new clinical entity. Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci. 1997;42:1458-1468. [Cited in This Article: ] |
| 4. | Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-698. [Cited in This Article: ] |
| 5. | Okazaki K. Autoimmune pancreatitis is increasing in Japan. Gastroenterology. 2003;125:1557-1558. [Cited in This Article: ] |
| 6. | Kim KP, Kim MH, Lee SS, Seo DW, Lee SK. Autoimmune pancreatitis: it may be a worldwide entity. Gastroenterology. 2004;126:1214. [Cited in This Article: ] |
| 7. | Sutton R. Autoimmune pancreatitis--also a Western disease. Gut. 2005;54:581-583. [Cited in This Article: ] |
| 8. | Liu QD, Zhou NX. Advances of diagnosis and treatment for autoimmune pancreatitis. Zhonghua Yixue Zazhi. 2007;87:1438-1440. [Cited in This Article: ] |
| 9. | Liu QD, Cai SW, Zhang WZ, Zhou NX. Autoimmume pancreatitis-jaundice-enlargement of pancreatic head-thickening of biliary duct wall-abnormality of CA19-9 level. Zhonghua Yixue Zazhi. 2007;87:640-642. [Cited in This Article: ] |
| 10. | Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237:853-858; discussion 858-859. [Cited in This Article: ] |
| 11. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694-2699. [Cited in This Article: ] |
| 12. | Nakazawa T, Ohara H, Sano H, Ando T, Imai H, Takada H, Hayashi K, Kitajima Y, Joh T. Difficulty in diagnosing autoimmune pancreatitis by imaging findings. Gastrointest Endosc. 2007;65:99-108. [Cited in This Article: ] |
| 13. | Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, Cavallini G. Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1-13. [Cited in This Article: ] |
| 14. | Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12:2487-2496. [Cited in This Article: ] |
| 15. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. [Cited in This Article: ] |
| 16. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [Cited in This Article: ] |
| 17. | Chang MC, Chang YT, Tien YW, Liang PC, Jan IS, Wei SC, Wong JM. T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem. 2007;53:1700-1705. [Cited in This Article: ] |
| 18. | Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, Colvin RB, Lauwers GY. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006;30:1537-1545. [Cited in This Article: ] |
| 19. | Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264-1269. [Cited in This Article: ] |
| 20. | Liu QD, Zhou NX, Zhang WZ, Wang MQ. Diagnosis and management of regional portal hypertension. Chin J Dig Dis. 2005;6:87-92. [Cited in This Article: ] |
| 21. | Mino-Kenudson M, Lauwers GY. Histopathology of autoimmune pancreatitis: recognized features and unsolved issues. J Gastrointest Surg. 2005;9:6-10. [Cited in This Article: ] |
| 22. | Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all 'chronic pancreatitis'? Am J Surg Pathol. 2003;27:110-120. [Cited in This Article: ] |
| 23. | Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, Brennan MF, Conlon K. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg. 2003;7:129-137; discussion 137-139. [Cited in This Article: ] |
| 24. | Chari ST, Echelmeyer S. Can histopathology be the “Gold Standard” for diagnosing autoimmune pancreatitis? Gastroenterology. 2005;129:2118-2120; discussion 2120. [Cited in This Article: ] |
| 25. | Suda K, Takase M, Fukumura Y, Ogura K, Ueda A, Matsuda T, Suzuki F. Histopathologic characteristics of autoimmune pancreatitis based on comparison with chronic pancreatitis. Pancreas. 2005;30:355-358. [Cited in This Article: ] |
| 26. | Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670-2676. [Cited in This Article: ] |
| 27. | Zamboni G, Luttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Kloppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552-563. [Cited in This Article: ] |
| 28. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [Cited in This Article: ] |
| 29. | Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683-687. [Cited in This Article: ] |
| 30. | Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613-625. [Cited in This Article: ] |
| 31. | Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23-28. [Cited in This Article: ] |
| 32. | Taguchi M, Aridome G, Abe S, Kume K, Tashiro M, Yamamoto M, Kihara Y, Nakamura H, Otsuki M. Autoimmune pancreatitis with IgG4-positive plasma cell infiltration in salivary glands and biliary tract. World J Gastroenterol. 2005;11:5577-5581. [Cited in This Article: ] |
| 33. | Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463-471. [Cited in This Article: ] |
| 34. | Khalid A, Nodit L, Zahid M, Bauer K, Brody D, Finkelstein SD, McGrath KM. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493-2500. [Cited in This Article: ] |
| 35. | Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP and MRI findings in 9 patients with autoimmune pancreatitis. World J Gastroenterol. 2006;12:2919-2922. [Cited in This Article: ] |
| 36. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [Cited in This Article: ] |
| 37. | Choi EK, Kim MH, Lee TY, Kwon S, Oh HC, Hwang CY, Seo DW, Lee SS, Lee SK. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35:156-161. [Cited in This Article: ] |
| 38. | Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Hishima T. Pancreatic cancer with a high serum IgG4 concentration. World J Gastroenterol. 2006;12:6225-6228. [Cited in This Article: ] |
| 39. | Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626-631. [Cited in This Article: ] |
| 40. | Choi EK, Kim MH, Kim JC, Han J, Seo DW, Lee SS, Lee SK. The Japanese diagnostic criteria for autoimmune chronic pancreatitis: is it completely satisfactory? Pancreas. 2006;33:13-19. [Cited in This Article: ] |
| 41. | CzakoL , Hegykozi E, Polinkas A, Lonovics J. Autoimmune pancreatitis: functional and morphological recovery after steroid therapy. World J Gastroenterol. 2006;12:1810-1812. [Cited in This Article: ] |