Published online Jan 28, 2008. doi: 10.3748/wjg.14.607
Revised: November 6, 2007
Published online: January 28, 2008
AIM: To study the expression of suppressor of cytokine signaling-1 (SOCS-1) in the liver tissues of chronic hepatitis B (CHB) and the clinical significance of this expression.
METHODS: The expression of SOCS-1 in liver tissues of 45 cases of CHB was investigated by immunohistochemical staining, and its correlations with inflammation grades and fibrosis stage were analyzed by SPSS statistics software.
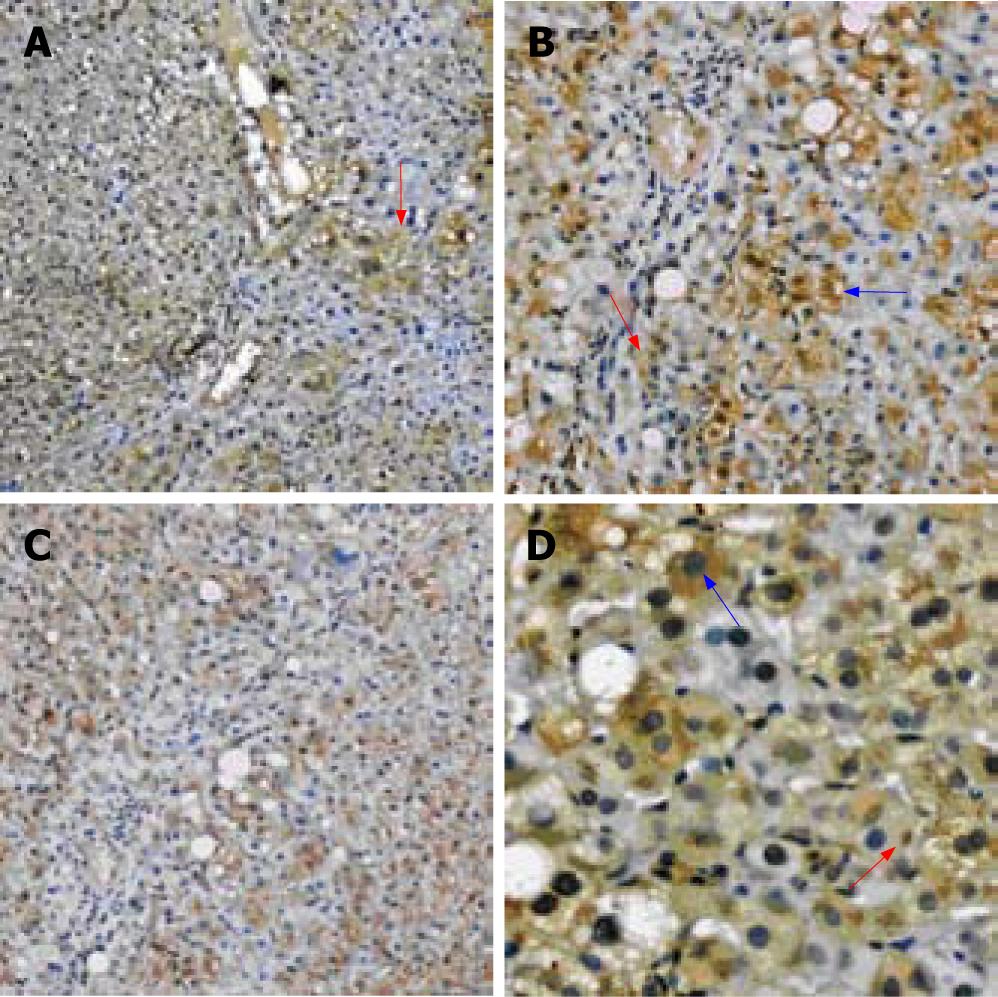
RESULTS: The result showed SOCS-1 expressing could be observed in the liver tissue of CHB. The expression of SOCS-1 was mainly distributed near the portal area in the liver tissue of mild inflammation CHB group, and was diffusely distributed in the liver tissue of moderate and severe inflammation groups. SOCS-1 positive stains mainly appear in the hepatocytes, only a few of liver interstitial cells were involved. Inside the hepatocyte, SOCS-1 positive stains are mainly distributed in the plasma. Some of the staining was observed on the membrane. The inclusion bodies in the plasma of hepatocytes were observed occasionally. There were both obvious correlations between the expression of SOCS-1 and the inflammatory grade, and that between the expression of SOCS-1 and the fibrosis stage.
CONCLUSION: The distribution of SOCS-1 in the liver tissue of CHB is variable. This expression was correlated with the inflammation grade and fibrosis stage.
- Citation: Zhao ZX, Cai QX, Peng XM, Chong YT, Gao ZL. Expression of SOCS-1 in the liver tissues of chronic hepatitis B and its clinical significance. World J Gastroenterol 2008; 14(4): 607-611
- URL: https://www.wjgnet.com/1007-9327/full/v14/i4/607.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.607
Hepatitis B virus (HBV) infection is a leading cause of chronic liver diseases globally. The exact mechanism by which HBV induces liver damage is still unknown. The suppressor of cytokine signaling (SOCS) family, which consists of SOCS-1 to 7 and cytokine-inducible SH2-containing protein (CIS), has been proved in the negative feedback regulation of janus kinase-signal transducer and activator of transcription (JAK/STAT) signal transduction pathway, which is induced by cytokine signaling[1–4]. SOCS-1 has been implied in the inhibition of inflammation of human body[5–8]. It also influences the inflammation and fibrosis of liver[9–11]. The recent research data also showed that SOCS-1 negatively regulated the response of chronic hepatitis C (CHC) to a-interferon (IFN-α)[1213]. Methylation of SOCS-1 is implicated to be correlated with carcinoma, including hepatocarcinoma, pancreatic cancer, multiple myeloma and myeloid leukemia[14–19]. Expression of SOCS-1 was regulated by many factors. Cytokines induced by inflammation upgrade the expression of SOCS-1[20–23]. Recent research data showed that, SOCS-1 transcripts in the livers of CHC were significantly higher than controls[12]. Therefore the objective of this study was to explore weather and how SOCS-1 expression in the liver tissues of chronic hepatitis B (CHB) patients and its relationship with inflammation grade and fibrosis stage of the livers.
Biopsied liver specimens were obtained from 45 CHB patients (44 males and 1 females) hospitalized in the infectious department of the third affiliated hospital of Sun Yat-San University to accept liver biopsy from August 2005 to November 2006. All cases were stratified into 3 groups (G2 to G4, 15 cases each group) according to the modified criteria for grading and staging of chronic hepatitis from hepatitis meeting consensus in 2000 of china.
All cases were HBsAg positive. Patients with infection of hepatitis virus of A, C, D, E, malignancy or antivirus therapeutic history were excluded.
Rabbit anti-human SOCS-1 monoclonal antibody (sc7006) was purchased from Santa Cruz Biotechnology. Diaminobenzidine (DAB) and Streptavidin Biotin Complex (SABC) were purchased from BOSTER Biotechnology in Wuhan.
The clinical data including age, gender, clinical history, liver function tests results done from 3 d before to 3 d after the biopsy and the evaluation results of liver pathology accorded to the modified criteria for grading and staging of chronic hepatitis were collected for analysis.
The routine paraffin-embedded liver tissue specimens were used. Antigen retrieval was performed by microwaving for 10 min at 90°C using Dako target retrieval solution (Dako, Carpinteria, CA, USA)[24]. The immunohistochemistry associated with anti-SOCS-1 was performed as described previously[25]. For a negative control, the primary antibody was replaced with non-immune normal mouse IgG.
The immunohistochemistry staining was evaluated by the staining area and density as described previously[26]. For the staining area, specimens without staining area score 0. Specimens with staining area smaller than 1/3 of the area observed score 1. The one larger than 2/3 scores 3. That from 1/3 to 2/3 score 2. For the staining density, light staining ones score 1, dark staining ones scored 2. The final score was the sum of area score and density score.
The SPSS 13.0 was used for the statistical analysis. Rank test was used to analyze the category rank data. Spearman non-parameter correlation analysis was used to analyze the rank associated data. Probabilities of P < 0.05 were assumed as statistically significant.
Under microscope, the positive staining for SOCS-1 was observed focally distributed. In the liver tissue of mild CHB group, the positive staining is mainly distributed near the portal area (Figure 1A). In the moderate and severe group, the positive staining is diffusely distributed (Figure 1B).
The positive staining for SOCS-1 mainly involves the hepatocytes, only a few of liver interstitial cells are involved (Figure 1C). The positive staining for SOCS-1 observed in the involved cell is mainly distributed in the plasma. Some of the staining was observed on the membrane. The inclusion bodies in the plasma of hepatocytes were observed occasionally (Figure 1D).
Evaluation of the staining for SOCS-1 in the mild hepatitis B group shows that 2 cases scored 0, 2 cases scored 2, 5 cases scored 3 and 6 cases scored 4. Evaluation of the staining for SOCS-1 in the moderate hepatitis B group shows that 1 case scored 2, 8 cases scored 3, 3 cases scored 4, 3 cases scored 5. Evaluation of the staining for SOCS-1 in the severe hepatitis B group shows that 2 cases scored 3, 7 cases scored 4, 6 cases scored 5 (Table 1).
| Evaluation of immunohistochemistry | |||||||
| 0 | 2 | 3 | 4 | 5 | Up | ||
| Group | G2 | 2 | 2 | 5 | 6 | 0 | 15 |
| G3 | 0 | 1 | 8 | 3 | 3 | 15 | |
| G4 | 0 | 0 | 2 | 7 | 6 | 15 | |
| Up | 2 | 3 | 15 | 16 | 9 | 45 | |
The Spearman correlation analysis was used to analyze the relationship between the expression of SOCS-1 and the pathologic degree. The result showed that the expression of SOCS-1 is positive correlated with the inflammatory degree and extent of the fibrosis stage (P = 0.001 and P = 0.04) (Table 2).
| Grade | Stage | ||
| Correlation | 0.493 | 0.308 | |
| SOCS-1 scores | P value | 0.001 | 0.04 |
| n | 45 | 45 |
The correlation between the expression of SOCS-1 and the liver function tests was analyzed by Spearman correlation analysis. The results showed that no correlation was observed (Table 3).
| mean ± SD | With SOCS-1 | ||
| Correlation | P-value | ||
| ALT (U/L) | 102.78 ± 108.302 | -0.077 | 0.617 |
| AST (U/L) | 86.89 ± 77.479 | 0.081 | 0.598 |
| ALP (U/L) | 82.11 ± 26.551 | -0.293 | 0.051 |
| GGT (U/L) | 137.47 ± 256.025 | 0.133 | 0.386 |
| ALB (G/L) | 42.276 ± 4.7947 | -0.058 | 0.704 |
| GLB (G/L) | 29.520 ± 4.1241 | -0.13 | 0.393 |
| TBIL (mol/L) | 22.724 ± 20.4962 | -0.084 | 0.583 |
| DBIL (&mgr;mol/L) | 7.950 ± 8.3926 | -0.008 | 0.961 |
| CHE (U/L) | 5771.36 ± 2350.354 | -0.026 | 0.86 |
The exact mechanism of CHB has not been clarified completely. By far, we have known that both the replication of HBV and the immune response of the host play important roles in hepatitis B. SOCS-1 is an important suppressor of cytokine signaling pathway in the serial reactions of immune response of host. SOCS-1 transcripts have been found in thymus cells, lung, liver, spleen, testis, and peripheral blood monocytes of mouse and human being by polymerase chain reaction (PCR)[122127].
Imanaka K compared the expression of SOCS-1 in the liver tissues of 21 cases of CHC patients and 9 control patients by quantitative reverse transcription-polymerase chain reaction (RT-PCR), and found that the transcripts of SOCS-1 in the liver tissues of CHC were significantly higher than controls[12]. The expression of SOCS-1 in the livers of CHC patients implied that it might associate with the inflammation and fibrosis of the livers, yet little has been known about whether and how it expresses in the liver tissues of CHB patients and its relationship with the mechanism of CHB.
The findings herein showed that there were variable expressions of SOCS-1 in the liver tissue of CHB. The expression of SOCS-1 was focally distributed. It was mainly distributed near the portal area in the livers of mild inflammatory CHB patients, and was diffusely distributed in the moderate and severe ones. As we know, inflammatory injure of the livers of CHB patients was mainly distributed near the portal area in the livers of mild inflammatory CHB patients, and was diffusely distributed in the moderate and severe inflammatory CHB patients. The findings suggested that expression of SOCS-1 in the livers of CHB patients accorded with the inflammation of the livers. This indicated that the expression of SOCS-1 was more or less correlated with the inflammation.
Research showed that SOCS-1 is expressed mainly in the hepatocytes and rarely in the interstitial cell. It may be due to the fact that inflammation in the livers of CHB patients mainly involves the hepatocytes. The signaling pathway in the hepatocytes was especially active, so that the expression of SOCS-1 in these cells was elevated. This finding showed that distribution of SOCS-1 in the livers of CHB patients was mainly in hepatocytes due to the influences of inflammation.
The molecular biological data shows that SOCS-1 is synthesized and executes its biological function in the cell plasma. Our results showed that no SOCS-1 was found in the nucleolus and the positive staining mainly seen in hepatocytes plasma. This indicated our findings complied with the previous reports.
Large amount of experiments show that SOCS-1 was an important inhibitor of inflammation. It inhibits the signaling pathway of many cytokines such as interleukin-2 (IL-2), IL-3, IL-4, IL-6, IFN-α, IFN-β, IFN-γ, tumor necrosis factor-α (TNF-α) and so on[21–23].
Naka et al found that mice lacking SOCS-1 would die within 3 wk of birth with extensive fatty degeneration of the liver and monocytic infiltration of several organs. His experiment demonstrated that SOCS-1 inhibited the inflammation[28].The new born mice with injection of large amount of IFN-γ showed the same phenotype with mice lacking SOCS-1, which could be contradicted by injection of anti-IFN-γ antibody. It suggested that the hypersensitivity of the mice to IFN-γ caused that inflammatory syndrome. SOCS-1 was a key negative regulator of gamma interferon action in vivo[29] and can inhibited the extent of inflammation.
The recent finding also showed that SOCS-1 protected oligodendrocytes from demyelination effects of interferon-gamma[30].
On the other hand, expression of SOCS-1 has been demonstrated to be induced by IFN-γ, Erythropoietin (EPO), Granulocyte Colony Stimulating Factor (G-CSF), Granulocyte-macrophage colony stimulating factor (GM-CSF), IL-2, IL-3, IL-4, IL-6, Leukemia inhibitor factor (LIF), growth hormone (GH), prolactin (PRL) and so on[20–23]. It referred to that inflammation could induce expression of SOCS-1.
The finding herein showed that expression of SOCS-1 was correlated with the inflammation grade of CHB patients. The reason might be that the inflammatory injure in the livers of the CHB patient induced the expression of SOCS-1. While SOCS-1 was not large enough to inhibit the inflammatory injure that have been occurred. But no significant relation between the expression of SOCS-1 and the liver function tests was observed. It may due to that liver function didn’t always accord with the pathological changes.
There were some reports that SOCS-1 can also inhibit liver fibrosis of the CHB patients. Yoshida examined SOCS-1 gene methylation in more than 200 cases of patients with chronic liver diseases and found that the severity of liver fibrosis is strongly correlated with SOCS-1 gene methylation[10]. Our finding showed that expression of SOCS-1 was correlated with the fibrosis stage of CHB patients. But the correlation is low and it is not easy to tell if it affects the progression of fibrosis directly or indirectly through negatively inhibiting the extent of inflammation by our results. Further research is needed to concern the relation between SOCS-1 expression and liver fibrosis.
Hepatitis B virus (HBV) infection is a leading cause of chronic liver diseases globally. The exact mechanism by which HBV induces liver damages is still unknown. The suppressor of cytokine signaling (SOCS) family, which consists of SOCS-1 to 7 and cytokine-inducible SH2-containing protein (CIS), has been proved in the negative feedback regulation of JAK/STAT signal transduction pathway, which is induced by cytokine. SOCS-1 was discovered by three different groups in 1997. It was named according to its feedback regulation of the cytokine signaling pathway. The former studies showed that SOCS-1 inhibited inflammation, fibrosis and carcinoma, and negatively regulated the response of chronic hepatitis C to IFN-α. It can be upgraded by cytokines induced by inflammation. But little has been known about whether and how SOCS-1 expresses in the liver tissues of CHB patients and its relationship with the mechanism of chronic hepatitis B (CHB).
There has been a considerable interest over recent years in the significance of SOCS-1 in signaling pathway. The interactions between SOCS-1 and some cytokines have been well explored. And the effect of SOCS-1 on inflammation and malignancy were the hotspots.
We described the distribution of SOCS-1 in the liver tissue of CHB by immunohistochemistry and performed correlation analysis on the expression of SOCS-1 with the inflammatory grade as well as the fibrosis stage and found positive correlation in both for the first time.
The research stated the distribution of SOCS-1 in the liver tissue of CHB. It is significant for the further statement of the mechanism of CHB and helpful for improvement of the management of CHB.
SOCS-1: SOCS-1 was discovered by three different groups in 1997. It was named according to its feedback regulation of the cytokine signaling pathway; 2. JAK/STAT signal transduction pathway: JAK/STAT signal transduction pathway is a signaling conduction pathway. It consisted of JAK and STAT. The mechanism includes phosphorylation of JAK, activation and aggregation of STAT.
The paper seems innovative. Zhao et al investigated the expression of SOCS-1 in the liver of patients chronically infected with HBV by immunohistochemistry and performed correlation analysis on the expression of SOCS-1 with the inflammatory grade as well as the fibrosis stage and found positive correlation for the first time. The experiment was well designed and plentitude of clinical data. Some interesting observations were obtained. All of these are significant for the further statement of the mechanism of CHB.
| 1. | Naka T, Fujimoto M, Kishimoto T. Negative regulation of cytokine signaling: STAT-induced STAT inhibitor. Trends Biochem Sci. 1999;24:394-398. |
| 2. | Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114-119. |
| 3. | Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, Mattfeldt T, Barth TF, Möller P. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Verh Dtsch Ges Pathol. 2006;90:210-215. |
| 4. | Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pità O, Leung DY, Howell MD. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984-992. |
| 5. | Yoshimura A, Mori H, Ohishi M, Aki D, Hanada T. Negative regulation of cytokine signaling influences inflammation. Curr Opin Immunol. 2003;15:704-708. |
| 6. | Fujimoto M, Tsutsui H, Xinshou O, Tokumoto M, Watanabe D, Shima Y, Yoshimoto T, Hirakata H, Kawase I, Nakanishi K. Inadequate induction of suppressor of cytokine signaling-1 causes systemic autoimmune diseases. Int Immunol. 2004;16:303-314. |
| 7. | Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677-687. |
| 8. | Chen Y, Chong MM, Darwiche R, Thomas HE, Kay TW. Severe pancreatitis with exocrine destruction and increased islet neogenesis in mice with suppressor of cytokine signaling-1 deficiency. Am J Pathol. 2004;165:913-921. |
| 9. | Lavon I, Sheinin T, Meilin S, Biton E, Weksler A, Efroni G, Bar-Joseph A, Fink G, Avraham A. A novel synthetic cannabinoid derivative inhibits inflammatory liver damage via negative cytokine regulation. Mol Pharmacol. 2003;64:1334-1341. |
| 10. | Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y, Sata M, Nagai H, Yoshimura A. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med. 2004;199:1701-1707. |
| 11. | Zhao F, Pan W, Liang YB, Tang H, Chen ZB, Lin MX, Yang XY, Zhou WX, Ma ZF. Study on the expression of supressors of cytokine signaling-1 in liver and spleen of septic mice. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 19:606-609. |
| 12. | Imanaka K, Tamura S, Fukui K, Ito N, Kiso S, Imai Y, Naka T, Kishimoto T, Kawata S, Shinomura Y. Enhanced expression of suppressor of cytokine signalling-1 in the liver of chronic hepatitis C: possible involvement in resistance to interferon therapy. J Viral Hepat. 2005;12:130-138. |
| 13. | Yang HP, Dai M, Zhang DH. Progress of study on suppressor of cytokine signaling-1 - review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:437-440. |
| 14. | Hatirnaz O, Ure U, Ar C, Akyerli C, Soysal T, Ferhanoglu B, Ozçelik T, Ozbek U. The SOCS-1 gene methylation in chronic myeloid leukemia patients. Am J Hematol. 2007;82:729-730. |
| 15. | Tischoff I, Hengge UR, Vieth M, Ell C, Stolte M, Weber A, Schmidt WE, Tannapfel A. Methylation of SOCS-3 and SOCS-1 in the carcinogenesis of Barrett's adenocarcinoma. Gut. 2007;56:1047-1053. |
| 16. | Nomoto S, Kinoshita T, Kato K, Otani S, Kasuya H, Takeda S, Kanazumi N, Sugimoto H, Nakao A. Hypermethylation of multiple genes as clonal markers in multicentric hepatocellular carcinoma. Br J Cancer. 2007;97:1260-1265. |
| 17. | Komazaki T, Nagai H, Emi M, Terada Y, Yabe A, Jin E, Kawanami O, Konishi N, Moriyama Y, Naka T. Hypermethylation-associated inactivation of the SOCS-1 gene, a JAK/STAT inhibitor, in human pancreatic cancers. Jpn J Clin Oncol. 2004;34:191-194. |
| 18. | Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784-2788. |
| 19. | Fukushima N, Sato N, Sahin F, Su GH, Hruban RH, Goggins M. Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br J Cancer. 2003;89:338-343. |
| 20. | Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659-666. |
| 21. | Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113:2813-2819. |
| 22. | Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378-387. |
| 23. | Chen XP, Losman JA, Rothman P. SOCS proteins, regulators of intracellular signaling. Immunity. 2000;13:287-290. |
| 24. | Pillai G, Roberts H, Gatter K, Pezzella F. p53 expression in normal paraffin-embedded tissue using different antibodies and antigen retrieval buffer systems. Histopathology. 2003;42:83-87. |
| 25. | Sakuda S, Tamura S, Yamada A, Miyagawa J, Yamamoto K, Kiso S, Ito N, Imanaka K, Wada A, Naka T. Activation of signal transducer and activator transcription 3 and expression of suppressor of cytokine signal 1 during liver regeneration in rats. J Hepatol. 2002;36:378-384. |
| 26. | Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990;21:607-612. |
| 27. | Wang T, Secombes CJ. Rainbow trout suppressor of cytokine signalling (SOCS)-1, 2 and 3: Molecular identification, expression and modulation. Mol Immunol. 2008;45:1449-1457. |
| 28. | Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci USA. 1998;95:15577-15582. |
| 29. | Bullen DV, Darwiche R, Metcalf D, Handman E, Alexander WS. Neutralization of interferon-gamma in neonatal SOCS1-/- mice prevents fatty degeneration of the liver but not subsequent fatal inflammatory disease. Immunology. 2001;104:92-98. |
| 30. | Balabanov R, Strand K, Kemper A, Lee JY, Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J Neurosci. 2006;26:5143-5152. |