Published online Jun 28, 2008. doi: 10.3748/wjg.14.3841
Revised: April 29, 2008
Accepted: May 6, 2008
Published online: June 28, 2008
AIM: To explore portal hypertension and portosystemic shunts and to stage chronic liver disease (CLD) based on the pathophysiology of portal hemodynamics.
METHODS: Per-rectal portal scintigraphy (PRPS) was performed on 312 patients with CLD and liver angioscintigraphy (LAS) on 231 of them. The control group included 25 healthy subjects. We developed a new model of PRPS interpretation by introducing two new parameters, the liver transit time (LTT) and the circulation time between right heart and liver (RHLT). LTT for each lobe was used to evaluate the early portal hypertension. RHLT is useful in cirrhosis to detect liver areas missing portal inflow. We calculated the classical per-rectal portal shunt index (PRSI) at PRPS and the hepatic perfusion index (HPI) at LAS.
RESULTS: The normal LTT value was 24 ± 1 s. Abnormal LTT had PPV = 100% for CLD. Twenty-seven non-cirrhotic patients had LTT increased up to 35 s (median 27 s). RHLT (42 ± 1 s) was not related to liver disease. Cirrhosis could be excluded in all patients with PRSI < 5% (P < 0.01). PRSI > 30% had PPV = 100% for cirrhosis. Based on PRPS and LAS we propose the classification of CLD in 5 hemodynamic stages. Stage 0 is normal (LTT = 24 s, PRSI < 5%). In stage 1, LTT is increased, while PRSI remains normal. In stage 2, LTT is decreased between 16 s and 23 s, whereas PRSI is increased between 5% and 10%. In stage 3, PRSI is increased to 10%-30%, and LTT becomes undetectable by PRPS due to the portosystemic shunts. Stage 4 includes the patients with PRSI > 30%. RHLT and HPI were used to subtype stage 4. In our study stage 0 had NPV = 100% for CLD, stage 1 had PPV = 100% for non-cirrhotic CLD, stages 2 and 3 represented the transition from chronic hepatitis to cirrhosis, stage 4 had PPV = 100% for cirrhosis.
CONCLUSION: LTT allows the detection of early portal hypertension and of opening of transhepatic shunts. PRSI is useful in CLD with extrahepatic portosystemic shunts. Our hemodynamic model stages the evolution of portal hypertension and portosystemic shunts. It may be of use in the selection of patients for interferon therapy.
- Citation: Dragoteanu M, Balea IA, Dina LA, Piglesan CD, Grigorescu I, Tamas S, Cotul SO. Staging of portal hypertension and portosystemic shunts using dynamic nuclear medicine investigations. World J Gastroenterol 2008; 14(24): 3841-3848
- URL: https://www.wjgnet.com/1007-9327/full/v14/i24/3841.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3841
The most frequent causes of chronic liver disease (CLD) are viral infections, ethanol, autoimmune, enzymatic and metabolic disorders[12]. Cirrhosis is the final stage of CLD[3]. Liver biopsy is still an important diagnostic tool in CLD[4].
Portal hypertension is a major complication which appears during CLD evolution. It is defined as an increase of portal blood pressure over 5-10 mmHg. Portosystemic shunts open when the venous portal-liver gradient becomes higher than 10-12 mmHg[56].
There are inferior, superior, anterior and posterior portosystemic shunts which could communicate with either inferior or superior vena cava territories. The existence of shunts as well as their blood flow is correlated to the severity and prognosis of CLD[7–9].
Investigation of portal pressure and portosystemic shunts may be performed by invasive and non-invasive methods. Invasive techniques offer the most correct data because of the direct measure of portal pressure. They have however a limited clinical use because of risks and costs[10].
Among the non-invasive methods, ultrasonography and upper digestive endoscopy are those currently used. The main parameters measured by ultrasonography to evaluate the effects of increased blood pressure in the portal territory are the diameters of portal, splenic and superior mesenteric veins, together with spleen size and portal flow velocity. However, dilation of portal territory veins can be seen in only 50% of cases[1112] and only 35%-80% of cirrhotic patients present esophageal varices at upper digestive endoscopy.
Nuclear medicine offers noninvasive static and dynamic procedures to investigate portal hypertension in CLD and to estimate the existence of portosystemic shunts[13].
A classic method is the liver scintigraphy using labeled colloid (planar and SPECT), which offers data regarding portal hypertension by calculating the capture ratio between liver and spleen, respectively between the right and left liver lobes[1415]. Increased colloid capture in the bone marrow is characteristic for advanced stages of portal hypertension.
Per-rectal portal scintigraphy (PRPS) investigates the hemodynamic importance of portosystemic shunts. Radio-tracer absorbed from rectum passes through inferior mesenteric vein into portal vein-liver-right heart[16–18]. The per-rectal portal shunt index (PRSI) was introduced by the classic works of Shiomi and co-authors as the main parameter calculated at PRPS by analyzing the dynamic curves raised on liver and heart areas[1920].
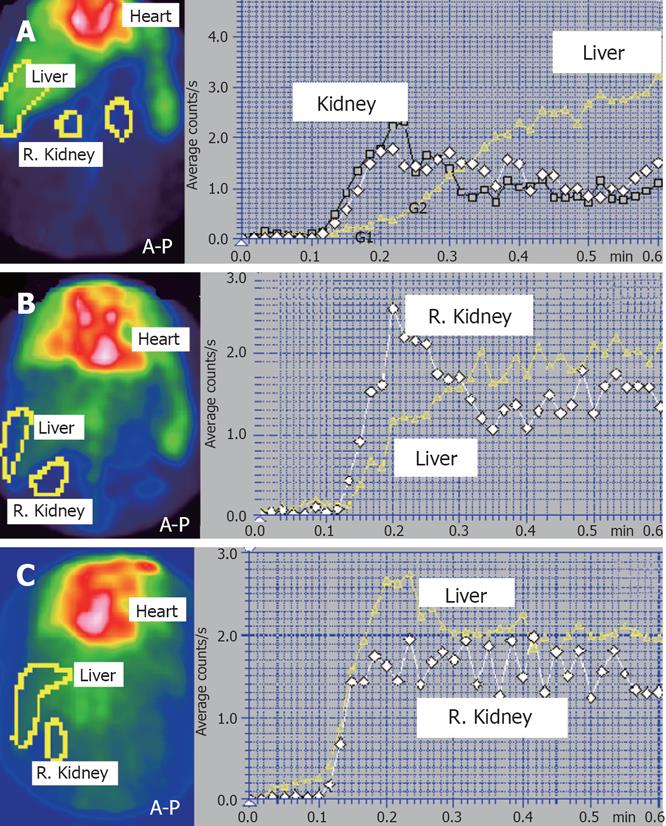
Liver angioscintigraphy (LAS) uses the hepatic perfusion index (HPI) to estimate the ratio between the hepatic artery inflow and total liver perfusion, arterial plus portal. Increased HPI (> 40%) in CLD shows the decrease of portal inflow with reactive increase of the flow through the hepatic artery by activation of the buffer response firstly described by Lautt[2122]. The decrease of portal inflow in advanced CLD is mainly determined by the quantity of blood deviated through portosystemic shunts[23–25]. In cirrhosis, HPI > 100% highlights the reversion of portal flow.
In this study we improved the interpretation of classical PRPS technique allowing a better characterization and staging of portal hypertension and portosystemic shunts.
PRPS with 99mTc-pertechnetate was performed in 312 consecutive patients there were 116 females and 196 males between 18 years and 80 years old. Their CLD diagnosis was based on clinical, laboratory, imaging and morphological data. The final diagnoses of the study population are shown in Table 1. The etiology of CLD in 291 patients is presented in Table 2.
| Type of disease | Number of patients |
| Steatosis | 17 |
| Chronic hepatitis | 69 |
| Cirrhosis | 202 |
| Unknown | 3 |
| CLD infirmed | 21 |
| Total | 312 |
| Etiology | Number of patients |
| Viral | 139 |
| Alcohol | 74 |
| Mix (viral + alcohol) | 6 |
| Unknown | 72 |
| Total | 291 |
LAS was performed on 231 patients with CLD, randomly selected from the 312 explored by PRPS. There were 141 males and 90 females, between 22 years and 77 years old.
Two hundred and four of the 312 patients who underwent PRPS were also investigated by upper digestive endoscopy: 175 of them had cirrhosis. One hundred and eleven of the cirrhotic patients who presented with esophageal varices at upper digestive endoscopy were followed up for 6 mo after investigation.
The control group for PRPS included 25 healthy subjects, 11 females and 14 males, between 18 years and 80 years old. The control group for LAS was composed of 25 healthy subjects, 10 females and 15 males, between 20 years and 78 years old.
PRPS was also performed on one group of 12 patients with complete thrombosis of portal vein in order to calculate RHLT and to compare its values with those in healthy subjects. This group included 6 females and 6 males, between 34 years and 73 years old.
All the patients were fasted for at least 12 h before LAS and PRSI.
Nuclear medicine investigations were made by using a SPECT Orbiter Siemens gamma-camera with high-resolution, low-energy, parallel collimator connected to a Power Macintosh computer, using ICON dedicated software.
For PRPS we used 99mTc-pertechnetate eluted from Drygen generators (General Electric-Amersham, UK). The colloid used for LAS was Hepatate (General Electric-Amersham, UK) labeled with 99mTc.
Two enemas were performed in each patient for PRPS: the first on the evening before the exam, and the second two hours prior to the examination.
The patients were positioned at PRPS with the camera detector in the anterior view including the liver and heart areas. A solution containing 2 milliliters of 99mTc-pertechnetate (296-370 MBq) was introduced into the upper part of the rectum, followed by 15 milliliters of air under pressure. Serial scintigrams were recorded every 2 s for 3 min. Radioactivity curves were built on liver and heart areas to show the dynamics of radio-tracer absorbed from the rectum.
LAS was performed after antecubital i.v. bolus injection of 370-440 MBq of 99mTc radio-colloid, with the patients lying down, in anterior-posterior view. Collimator area included abdomen and lower part of thorax. Sequential images were recorded 1/s for 1 min. Right kidney, right liver lobe and spleen dynamic curves were built. HPI was calculated using the Sarper's method[26].
The results were compared with clinical diagnosis, liver biopsy and upper digestive endoscopy. For statistical evaluation of PRSI we used the Kruskall-Wallis and Mann-Withney non-parametric tests.
At the beginning of the research we used for the first 100 patients the classic interpretation of PRPS, based on the calculation of PRSI for the global area of the liver[16]. All information was stored in the computer.
In the second stage we considered the possibility of acquiring more useful data in PRPS, by developing a new model of interpretation. Pertechnetate is not significantly captured by liver or heart, so that at PRPS the first-passage histograms built for these organs represent transit curves, not accumulation curves. This suggests the importance of time-related parameters. Transit type of PRPS liver and heart curves is highlighted by dynamic curves built on the inferior mesenteric vein area. These curves have the same ascending aspect as the histograms on liver and heart thereafter produced at first passage by the same radiotracer flow absorbed from the upper rectum.
We introduced LTT as a new parameter useful for the early phases of CLD where PRSI offers not enough information. LTT is the time interval between entrance into the liver and subsequent entrance into the right heart of the radiotracer absorbed from the upper rectum, after passing through the mesenteric and portal veins. LTT was separately measured for each liver lobe as the time interval between the liver and heart dynamic histograms. The normal value is 24 ± 1 s. This parameter is useful for patients without extra-hepatic shunts. In patients with portosystemic extrahepatic shunts, LTT cannot be correctly determined by PRPS because the tracer absorbed from the rectum may arrive at the right heart faster by passing through shunts than following the physiological pathway.
PRSI equal to 10% corresponds to LTT equal to 16 ± 1 s. LTT decreased between 16 s and 23 s corresponds to a PRSI increased up to 10% and approximates the interval in which only the transhepatic shunts are open. A PRSI equal to 30% corresponds to a mean time of 8 s between the liver and heart curves, but this interval does not have the significance of LTT because of the flow passing through the extra-hepatic portosystemic shunts, which arrives at the heart faster than the tracer passing through the liver.
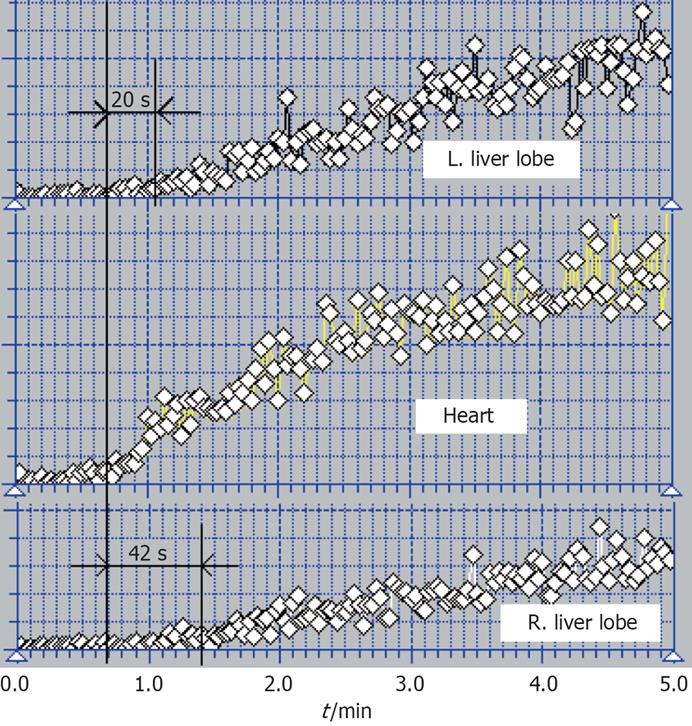
Liver areas perfused only through the hepatic artery may be seen in cirrhosis. One lobe or both have, in such cases, abolished or insignificant portal inflow and the time between PRPS curves on the heart and on the area(s) without portal inflow has a maximum value, equal to RHLT. This is a constant time interval (not related to CLD) between the entrance of the tracer into the right heart and its subsequent arrival to the liver following the route: right heart-lungs-left heart-aorta-hepatic artery. We measured RHLT in the patients with complete portal thrombosis as the time interval between heart and liver curves. In healthy controls, RHLT was measured as the time interval between the arrival of tracer into the right heart and the ascending inflexion on the liver histogram determined by the subsequent arrival of tracer through the hepatic artery. The value of RHLT was 42 ± 1 s for both methods.
We performed separate PRPS analyses of the two liver lobes, which are autonomous in relation to the blood inflow (Figure 1). No differences between the two lobes could be found in the control group. We compared PRPS curves for the two lobes in order to distinguish subtypes, respectively stages of portal hypertension evolution. Figure 1 shows the case of a cirrhotic patient in which time intervals between the heart histogram and the curves on the two liver lobes are different.
Our PRPS model is based on the two new time parameters, the classic calculation of PRSI and the separate evaluation of the two liver lobes.
The distribution for the 312 patients of the PRSI calculated using Shiomi’s formula is presented in Table 3.
| Per-rectal portal shunt index (%) | Number of patients (n) | Cirrhosis | Stage in our classification | |
| 0-5 | 65 | 0 | Stages 0 & 1 | |
| 5-10 | 13 | 5 | Stage 2 | |
| 10-20 | 42 | 80 | 43 | Stage 3 |
| 20-30 | 38 | |||
| 30-40 | 17 | 154 | 154 | Stage 4 |
| 40-50 | 10 | |||
| 50-60 | 11 | |||
| 60-70 | 10 | |||
| 70-80 | 18 | |||
| 80-90 | 40 | |||
| 90-100 | 48 | |||
| Total | 312 | 202 | ||
There were no correlations of PRPS and LAS parameters with sex or age (P < 0.01).
As many as 202 of 312 patients investigated with the PRPS were diagnosed as having a cirrhosis. One hundred and seventy-five of these underwent upper digestive endoscopy. Twenty seven patients with advanced cirrhosis (all of them with PRSI > 30% at PRPS) could not be explored by endoscopy as deemed too risky. Using Child-Pugh classification, the 175 cirrhotic patients investigated by upper digestive endoscopy were classified as follows: 99 in class A, 38 in class B and 38 in class C.
Only 16 patients from the 93 with PRSI between 5% and 30% had esophageal varices. Thirty two additional patients with PRSI < 30% but without varices were also diagnosed with cirrhosis. As a result, we had a total number of 48 cirrhotic patients with PRSI < 30%. Five patients with cirrhosis had discordant low PRSI values ranging from 5% to 10%, but no cirrhotic patient had normal PRSI (< 5%). All the patients with PRSI > 30% had cirrhosis, so we used PRSI = 30% as an upper limit value for chronic hepatitis.
The PRSI was significantly higher in cirrhotic patients than in chronic hepatitis (P < 0.01). The median value for PRSI was 5% for the control group, 5% for the patients with steatosis, 6% for the patients with chronic hepatitis and 73.5% for the cirrhotic patients. The sums of ranks for the PRSI values based on Kruskall-Wallis test for the healthy subjects and stages of CLD are shown in Table 4 (P = 0.000). Using the Mann-Withney test we showed that there was a significant statistical difference between PRSI for patients with chronic hepatitis and the controls (P = 0.0003), between patients with cirrhosis and those with chronic hepatitis (P = 0.0000) and respectively between patients with cirrhosis and controls (P = 0.0000).
| Disease | Number of patients (n) | Sum of ranks |
| CLD infirmed | 21 | 759.5 |
| Steatosis | 17 | 1201.5 |
| Chronic hepatitis | 69 | 5004.5 |
| Cirrhosis | 202 | 40929.5 |
One hundred and eleven patients with esophageal varices and PRSI > 30% were followed up for 6 mo; 51 of these had experienced previous upper digestive bleeding. During the follow up, 17 of these patients had an episode of upper digestive bleeding (11 as first time, 6 as recurrence). All our patients with upper digestive bleeding had PRSI > 70% (mean of 88%). The 94 patients from the group of 111 with esophageal varices who had not upper digestive bleeding during the follow up had a mean value of PRSI equal to 46.75%. We had no patients with upper digestive bleeding among those with PRSI < 30%, even if they had esophageal varices at upper digestive endoscopy.
These results show the diagnostic value of the classic parameter PRSI. A PRSI < 5% had a NPV = 100% for cirrhosis, while a PRSI > 30% had PPV = 100% for cirrhosis. A PRSI > 70% was associated with a high risk of upper digestive bleeding (P < 0.01), while with a PRSI < 30% no variceal bleeding was encountered. However, using only PRSI is not always possible to make the differential diagnosis between chronic hepatitis and cirrhosis or between chronic hepatitis and healthy subjects.
Using LTT parameter originally introduced by us at PRPS we were able to find more data about the early stages of portal hypertension, when PRSI cannot offer enough information. In healthy subjects, LTT was 24 ± 1 s. The distribution of LTT in patients with PRSI smaller than 10% (LTT > 16 s) is shown in Table 5. For the 27 non-cirrhotic CLD patients who had prolonged LTT (> 25 s), the median value was 27 s. LTT determined at PRPS has hemodynamic significance as it shows the time required at first-passage by the main part of portal inflow of radio-tracer to arrive to right heart through the liver.
| Per-rectal shunt index | Mean value of liver transit time (s) | Etiology | Number of patients (n) | Stage in our classification | |
| Right lob | Left lobe | ||||
| < 5% | 24 | - | 38 | Stage 0 | |
| 25 | 28 | viral | 10 | Stage 1a | |
| 31 | 24 | alcoholic | 6 | Stage 1b | |
| 31.5 | 29 | viral | 7 | Stage 1c | |
| viral + alcoholic | 4 | ||||
| 5%-10% | 19.5 | 13 | Stage 2 | ||
RHLT measured in the control group and in patients with complete portal thrombosis is 42 ± 1 s. We used it to identify cirrhotic patients with undetectable portal inflow to one liver lobe or both. Other causes of portal flow interruption (thromboses, compressions) were excluded by ultrasonography.
HPI was used in our study to show the reactive increase of arterial flow due to the decreasing of portal inflow (HPI > 40%) and to identify those cirrhotic patients with reversed portal flow (HPI > 100%).
Using the above shown data we propose a hemodynamic model in 5 steps to stage the evolution of portal hypertension and portosystemic shunts on physiopa-thological basis using nuclear medicine dynamic investigations (PRPS and LAS).
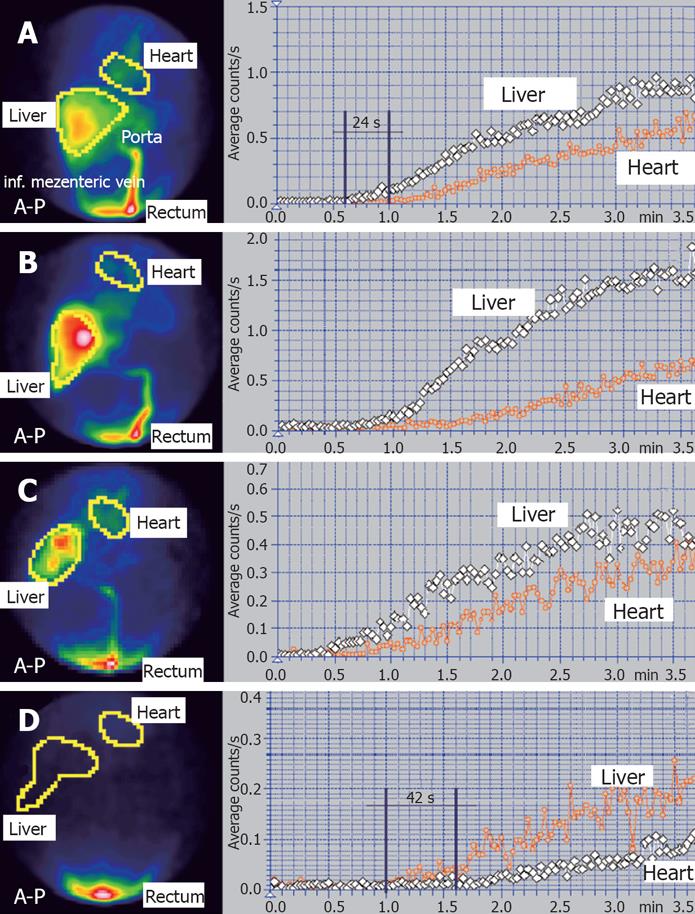
The 5 stages of portal hypertension and portosystemic shunts proposed by us are the following: (1) Stage 0 is normal: PRSI < 5%, LTT = 24 ± 1 s (Figure 2A), HPI < 40% (Figure 3A); (2) Stage 1 is characterized by PRSI < 5% (normal), but LTT is increased over 25 s at least on one lobe (Figure 2B). HPI is normal. For stage 1 we found 3 subtypes according to the liver lobe(s) with prolonged LTT: (a) Subtype 1a, with increased LTT for the left lobe, but with normal or slightly decreased LTT for the right lobe; (b) Subtype 1b, with increased LTT for the right lobe and with normal or slightly decreased LTT for the left lobe; (c) Subtype 1c, with increased LTT for the both lobes; (3) Stage 2 is characterized by decreased LTT for both lobes, between 16 s and 23 s. PRSI is slightly increased, between 5% and 10% (Figure 2C). HPI calculated at LAS is currently at the upper normal limit or slightly increased, up to 45%; (4) Stage 3 is characterized by a moderately increased PRSI, between 10%-30%. Time interval between the hepatic and heart curves is decreased between 8 s and 16 s, but it is no more equal to LTT due to the shunts. HPI is moderately increased, usually up to 50%-55%; (5) Stage 4 is characterized by PRSI > 30%. HPI is increased over 60%-70%. Liver curve precedes heart curve with less than 8 s or heart curve precedes liver curve (when PRSI > 50%). We found 4 subtypes of stage 4, according to the lobe(s) with undetectable or reversed portal flow: (a) Subtype 4a: heart curve precedes the hepatic histograms on both lobes with less than 42 s. Both lobes have portal inflow; (b) Subtype 4b: Cardiac curve precedes right liver lobe histogram with 42 s and the left liver lobe histogram with less than 42 s. There is still a portal flow to the left lobe, but the portal inflow to the right lobe is undetectable (Figure 1); (c) Subtype 4c: PRSI > 95%. Time between heart and both liver lobes curves is equal to RHLT = 42 s (Figure 2D). PRPS cannot detect portal inflow to any of the hepatic lobes. For subtypes 4a, 4b and 4c, the HPI is increased over 60%, but smaller than 100% (Figure 3B); (d) Subtype 4d: HPI at LAS is higher than 100% (Figure 3C). The time between PRPS heart curve and both liver lobes histograms is 42 s. Portal flow is reversed.
Stage 0 includes the subjects without CLD. There are no portal flow changes. In our study, stage 0 had NPV = 100% for CLD. The tracer absorbed at PRPS from the rectum reaches the liver through the physiological pathway (inferior mesenteric and portal veins).
In stage 1, it is possible to detect the earliest changes that affect either one lobe or both, determined by the increased resistance opposed by liver to portal inflow. Portal flow velocity decreases and LTT is consequently increased. In our study stage 1 had PPV = 100% for non-cirrhotic CLD. In this stage the transhepatic and extrahepatic shunts are not open and the arterial inflow is normal. We encountered subtype 1a in patients with chronic viral hepatitis, subtype 1b in alcoholic etiology and subtype 1c in viral and mix (viral + alcohol) etiologies.
Stage 2 theoretically corresponds to the dilation of part of transhepatic pathways between portal and hepatic veins as a result of the portal pressure which is increased at higher values than in stage 1. The blood passes faster through these dilated transhepatic shunts than through sinusoids[2728]. LTT is consequently decreased. Our threshold PRSI = 10% between stage 2 and stage 3 was selected based on the correspondence of this PRSI value with LTT = 16 ± 1 s. Extrahepatic shunts are not open and arterial inflow remains normal (hepatic artery buffer response is not activated). Stage 2 theoretically appears when the increased resistance opposed by liver to the portal inflow produces a higher portal pressure which is able to enlarge transhepatic pathways, but is not high enough to open extrahepatic shunts. As stage 1, stage 2 has PPV = 100% for CLD, but stage 2 includes not only chronic hepatitis, but also cirrhotic patients, showing a more advanced stage of portal hypertension than stage 1. 38.46% of our 13 patients in stage 2 had cirrhosis, the other 61.54% had chronic hepatitis.
A steady state between stage 1 and stage 2 may appear in cases with the resistance (and LTT) increased on one more affected liver lobe and with redirecting of an increased percentage of the portal inflow through the other lobe. In such cases, the lobe less affected may encounter opening of transhepatic shunts due to its higher portal inflow (with consequently slightly decreased LTT, like in stage 2), while the more affected lobe has prolonged LTT (characteristic for stage 1).
Stage 3 is theoretically characterized by the opening of extrahepatic shunts, added to the transhepatic dilated pathways already present from stage 2. Low flow extrahepatic shunts appear when the transhepatic shunts are no more able to compensate the higher values of portal pressure. The inferior per-rectal portosystemic shunts are in most cases the first extrahepatic shunts which open. The cause is the pressure gradient between inferior mesenteric vein and inferior vena cava territories, which is lower than the pressure gradient between portal vein and superior vena cava. The portal inflow to the liver decreases due to shunted flow and PRSI increases over 10%. A reactive increase of the arterial liver inflow due to the activation of the buffer mechanism of the hepatic artery is reflected at LAS by HPI > 40%. 53.75% of our 80 patients in stage 3 had cirrhosis, the other 46.25% had chronic hepatitis.
Stage 4 in our study had PPV = 100% for cirrhosis. Theoretically, stage 4 involves shunts open to the territory of superior vena cava, which has a higher diameter and increased flow[29]. PRPS shows diminished or abolished portal inflow to one or both liver lobes, with PRSI > 30%. In stage 4, the tracer absorbed at PRPS from the rectum usually arrives faster to the right heart (through the portosystemic shunts and caval veins) than to the liver through the physiological pathway. Inverted order of heart and liver curves may be thus seen in advanced cases, corresponding to PRSI > 50%. In patients with undetectable portal inflow to one or both lobes (subtypes 4b, 4c, 4d), the tracer absorbed from the rectum reaches those liver areas only through the hepatic artery and time interval between the heart and liver curves is equal to RHLT = 42 s. Subtype 4b appeared more frequently in alcoholic CLD. In our study, we did not have patients with abolished inflow to the left lobe and maintaining portal inflow to the right lobe, but such cases may theoretically exist.
Figure 1 shows a case where the interval between heart and left liver lobe curves was equal to 20 s (left lobe maintained a low portal inflow) while the time interval between heart and right liver lobe histograms was equal to RHLT = 42 s (the right lobe received tracer only through hepatic artery).
In our group, the number of patients in early stages (1 and 2) was lower than in advanced stages (3 and 4). This could be explained by the fact that few patients in early CLD stages were hospitalized and/or proposed for nuclear medicine dynamic liver investigations. Moreover, stage 2 is theoretically an intermediate stage in the natural history of CLD and of the portal hypertension, so it lasts a short time compared to the evolution of the disease.
Our experience confirmed that at PRSI > 70% the risk of upper digestive hemorrhage is increased[1730].
The classical parameter PRSI allows only a rough characterization of non-cirrhotic patients. PRSI gives no information about early increased resistance opposed by liver to the portal inflow or about the existence of transhepatic shunts, the first that open due to the portal hypertension. Another diagnostic limitation in using PRSI alone is the fact that there are patients with chronic hepatitis but who have normal PRSI, lower than 5%.
Our model using LTT besides PRSI allows a very early diagnosis of portal hypertension, represented by stages 1 and 2. Changes of liver dynamic resistance opposed to portal inflow could be shown in these early stages, at a moment when morphological effects on the portohepatic circulation are not detectable. The diagnosis of early hemodynamic changes determined by portal hypertension could be the basis for an appropriate therapy in a stage when the disease is reversible. Thus, we propose LTT as the main parameter of PRPS in the evaluation of early stages of portal hypertension. PRSI remains a very useful parameter for portal hypertension and portosystemic shunts in advanced stages of CLD.
Staging of portal hypertension has implications in the selection of patients for the treatment. Hemodynamic pathophysiology information offered by nuclear medicine dynamic investigations may improve also the selection of patients for interferon therapy.
Portal hypertension and portosystemic shunts are severe complications of chronic liver disease (CLD). Their evaluation could be considered a dynamic marker of the progression of the disease. Ultrasonography and upper digestive endoscopy are usually performed to evaluate their existence and hemodynamic importance. Nuclear medicine techniques like per-rectal portal scintigraphy (PRPS) and liver angioscintigraphy (LAS) can offer valuable supplementary information.
Doppler ultrasonography and MRI are continuously increasing their accuracy in exploring portal hypertension and portosystemic shunts. PRPS is usually performed to investigate the advanced stages of CLD. The classical PRPS parameter per-rectal portal shunt index (PRSI) is useful in cases with open portosystemic shunts. Our research improves the diagnosis possibilities of PRPS especially in early stages of CLD by introducing two new time parameters. The early diagnosis of CLD and the therapy (including the selection of patients for interferon therapy) may be improved using dynamic scintigraphy data.
We introduced two new parameters at PRPS, respectively liver transit time (LTT) and right heart to liver transit time (RHLT). LTT is useful in early stages of portal hypertension, before the opening of extrahepatic portosystemic shunts. LTT allows the diagnosis of early increase of liver resistance opposed to portal inflow and of the opening of transhepatic shunts. RHLT is useful in advanced CLD stages to detect liver areas missing portal inflow. We propose the classification of portal hypertension and portosystemic shunts in 5 hemodynamic stages, characteristic for the progression of the disease. We introduce the separate evaluation of the two liver lobes at PRPS, used to subtype the stages 1 and 4.
Using LTT as a basic parameter, PRSI allows the detection of early stages of portal hypertension, which are reversible under proper therapy. Our method can also distinguish between the 1st stage of portal hypertension, with increased resistance opposed by liver to portal inflow, without shunts, and the 2nd stage, characterized by the opening of transhepatic shunts. The new parameter RHLT and the hepatic perfusion index (HPI) calculated at LAS allow a better characterization of liver hemodynamics in advanced cirrhosis. We confirm the results of other studies showing that at PRSI > 70% the risk for upper digestive bleeding increases. The classification of portal hypertension and portosystemic shunts in 5 hemodynamic stages is useful for clinicians, in order to have a more accurate view of the patients with CLD. A better understanding of hemodynamic status of border-line cases between chronic hepatitis and cirrhosis may also improve the selection of patients for interferon therapy. Patients with PRSI between 5%-30% (stages 2 and 3 in our classification) require a precise evaluation in order to choose an adequate therapy. Correlation of dynamic nuclear medicine techniques with other non-invasive methods makes it possible to avoid liver biopsy for guiding the treatment in border-line patients between chronic hepatitis and cirrhosis. Dynamic follow-up of patients under interferon-treatment may be useful to adjust the therapy. Based on calculation of LTT, PRPS may be used to determine whether the early portal pressure reducing effect of anti-viral therapy is maintained in the long term, especially in sustained viral responders. It can be also helpful to evaluate whether long-term use of anti-viral therapy may delay the appearance and decrease the severity of portal hypertension manifestations.
Per-rectal portal scintigraphy (PRPS) is a dynamic nuclear medicine technique which investigates the existence and hemodynamic importance of portal hypertension and portosystemic shunts. A radiotracer introduced in the upper rectum is absorbed and follows the next pathway: inferior mesenteric vein-portal vein-liver-right heart. Dynamic curves built on liver and heart allow the calculation of specific parameters. Liver transit time (LTT) determined at PRPS is the time interval between entrance into the liver and subsequent entrance into the right heart of the radiotracer absorbed from the rectum, after passing through mesenteric and portal veins. Right heart to liver transit time (RHLT) represents at PRPS a constant time interval (not related to liver disease) between the entrance of the tracer into the right heart and its subsequent arrival to the liver following the next route: right heart-lungs-left heart-aorta-hepatic artery. Per-rectal portal shunt index (PRSI) is a parameter calculated at PRPS by analysis of dynamic curves built on liver and heart areas. Liver angioscintigraphy (LAS) is a dynamic nuclear medicine method based on i.v. antecubital administration of a radio-tracer and subsequent analyses of the liver dynamic curve which is determined at first passage by both hepatic artery and portal inflows of tracer. Hepatic perfusion index (HPI) is a parameter calculated at LAS which estimates the ratio between hepatic artery inflow and total liver perfusion, arterial plus portal.
This is a well done study probably not completely well presented where Dragoteanu and coworkers used per-rectal portal scintigraphy and liver angioscintigraphy in 312 and 231 CLD patients and 25 controls to calculate hepatic perfusion index (HPI) and other new hemodynamic parameters and classify portal hypertension and porto-caval shunts in 5 hemodynamic stages, which are specifically for the progression of CLD.
| 1. | Yip WW, Burt AD. Alcoholic liver disease. Semin Diagn Pathol. 2006;23:149-160. |
| 2. | Hui AY, Sung JJ. Advances in chronic viral hepatitis. Curr Opin Infect Dis. 2005;18:400-406. |
| 4. | Theise ND. Liver biopsy assessment in chronic viral hepatitis: a personal, practical approach. Mod Pathol. 2007;20 Suppl 1:S3-S14. |
| 5. | Blei AT. Portal hypertension and its complications. Curr Opin Gastroenterol. 2007;23:275-282. |
| 6. | Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. |
| 7. | D’Albuquerque LA, de Oliveira e Silva A, Pinto Junior PE, de Miranda MP, Genzini T, Gama-Rodrigues JJ. [Surgical treatment of portal hypertension in patients with liver cirrhosis]. Arq Gastroenterol. 1988;25:218-223. |
| 8. | Rice TL. Treatment of esophageal varices. Clin Pharm. 1989;8:122-131. |
| 9. | Wolff M, Hirner A. Current state of portosystemic shunt surgery. Langenbecks Arch Surg. 2003;388:141-149. |
| 10. | Whalley S, Puvanachandra P, Desai A, Kennedy H. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med. 2007;7:119-124. |
| 11. | Gorg C, Riera-Knorrenschild J, Dietrich J. Pictorial review: Colour Doppler ultrasound flow patterns in the portal venous system. Br J Radiol. 2002;75:919-929. |
| 12. | Badea R, Lupsor M, Stefanescu H, Nedevschi S, Mitrea D, Serban A, Vasile T. Ultrasonography contribution to the detection and characterization of hepatic restructuring: is the "virtual biopsy" taken into consideration? J Gastrointestin Liver Dis. 2006;15:189-194. |
| 13. | Dragoteanu M, Cotul SO, Tamas S, Piglesan C. Nuclear medicine dynamic investigations of diffuse chronic liver diseases and portal hypertension. Rom J Gastroenterol. 2004;13:351-357. |
| 14. | Mostbeck A, Kroiss A. [Nuclear-medical methods in hepatology]. Dtsch Z Verdau Stoffwechselkr. 1981;41:1-13. |
| 15. | Cotul S. [The current posture on radioisotope exploration in chronic diffuse hepatopathies]. Rev Med Interna Neurol Psihiatr Neurochir Dermatovenerol Med Interna. 1988;40:211-216. |
| 16. | Shiomi S, Kuroki T, Kurai O, Kobayashi K, Ikeoka N, Monna T, Ochi H. Portal circulation by technetium-99m pertechnetate per-rectal portal scintigraphy. J Nucl Med. 1988;29:460-465. |
| 17. | Chitapanarux T, Praisontarangkul OA, Thongsawat S, Pisespongsa P, Leerapun A. Per rectal portal scintigraphy as a useful tool for predicting esophageal variceal bleeding in cirrhotic patients. World J Gastroenterol. 2007;13:791-795. |
| 18. | Kawamura E, Habu D, Hayashi T, Oe A, Kotani J, Ishizu H, Torii K, Kawabe J, Fukushima W, Tanaka T. Natural history of major complications in hepatitis C virus-related cirrhosis evaluated by per-rectal portal scintigraphy. World J Gastroenterol. 2005;11:3882-3886. |
| 19. | Shiomi S, Sasaki N, Habu D, Takeda T, Nishiguchi S, Kuroki T, Tanaka T, Ochi H. Natural course of portal hemodynamics in patients with chronic liver diseases, evaluated by per-rectal portal scintigraphy with Tc-99m pertechnetate. J Gastroenterol. 1998;33:517-522. |
| 20. | Shiomi S, Kuroki T, Ueda T, Takeda T, Ikeoka N, Nishiguchi S, Nakajima S, Kobayashi K, Ochi H. Clinical usefulness of evaluation of portal circulation by per rectal portal scintigraphy with technetium-99m pertechnetate. Am J Gastroenterol. 1995;90:460-465. |
| 21. | Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol. 1985;249:G549-G556. |
| 22. | Gulberg V, Haag K, Rossle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatology. 2002;35:630-634. |
| 23. | Dragoteanu M, Cotul SO, Piglesan C, Tamas S. Liver angioscintigraphy: clinical applications. Rom J Gastroenterol. 2004;13:55-63. |
| 24. | Santambrogio R, Bruno S, Opocher E, Galeotti F, Zatta G, Grugni M, Macri M, Pisani A, Tarolo G, Spina G. Angioscintigraphic assessment of hemodynamic effects of penbutolol in cirrhotics with portal hypertension. A double-blind, randomized, controlled study. Hepatogastroenterology. 1990;37:398-402. |
| 25. | Zatta G, Santambrogio R, Boccolari S, Mana O, Gattoni F, Baldini U, Galeotti F, Opocher E, Spina GP, Tarolo GL. Angioscintigraphic assessment of arterial and portal liver blood flow: comparison with splanchnic angiography. Nuklearmedizin. 1987;26:83-86. |
| 26. | Sarper R, Tarcan YA. An improved method of estimating the portal venous fraction of total hepatic blood flow from computerized radionuclide angiography. Radiology. 1983;147:559-562. |
| 27. | Huet PM, Pomier-Layrargues G, Villeneuve JP, Varin F, Viallet A. Intrahepatic circulation in liver disease. Semin Liver Dis. 1986;6:277-286. |
| 28. | Chin N, Ohnishi K, Iida S, Nomura F. Role of intrahepatic portal-systemic shunts in the reduction of portal blood supply to liver cells in cirrhosis. Am J Gastroenterol. 1988;83:718-722. |
| 29. | Tiani C, Abraldes JG, Bosch J. Portal hypertension: pre-primary and primary prophylaxis of variceal bleeding. Dig Liver Dis. 2008;40:318-327. |
| 30. | Hartleb M, Boldys H, Rudzki K, Nowak A, Nowak S. Portal shunting in inferior mesenteric vein in cirrhosis: correlation with hemorrhage from esophageal varices. Am J Gastroenterol. 1994;89:863-867. |