Published online Jun 7, 2008. doi: 10.3748/wjg.14.3338
Revised: October 23, 2007
Accepted: October 30, 2007
Published online: June 7, 2008
Primary sclerosing cholangitis is a chronic cholestatic liver disease characterized by inflammation and fibrosis of the bile ducts, resulting in cirrhosis and need for liver transplantation and reduced life expectancy. The majority of cases occur in young and middle-aged men, often in association with inflammatory bowel disease. The etiology of primary sclerosing cholangitis includes immune-mediated components and elements of undefined nature. No effective medical therapy has been identified. The multiple complications of primary sclerosing cholangitis include metabolic bone disease, dominant strictures, bacterial cholangitis, and malignancy, particularly cholangiocarcinoma, which is the most lethal complication of primary sclerosing cholangitis. Liver transplantation is currently the only life-extending therapeutic alternative for patients with end-stage disease, although recurrence in the allografted liver has been described. A PSC-like variant attracting attention is cholangitis marked by raised levels of the immunoglobulin G4 subclass, prominence of plasma cells within the lesions, and steroid responsiveness.
- Citation: Silveira MG, Lindor KD. Clinical features and management of primary sclerosing cholangitis. World J Gastroenterol 2008; 14(21): 3338-3349
- URL: https://www.wjgnet.com/1007-9327/full/v14/i21/3338.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3338
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by fibrosing inflammatory destruction of the intrahepatic and/or extrahepatic bile ducts[1], leading to bile stasis, hepatic fibrosis, and ultimately to cirrhosis, end-stage liver disease, and need for liver transplantation. The majority of cases occur in association with inflammatory bowel disease (IBD), which often precedes the development of PSC[2]. The etiology of PSC is undefined, apart from an increasing body of evidence that points to an immunologic disturbance as a component of the disease. However, PSC lacks the features of a typical autoimmune disease and responds poorly, if at all, to typical immunosuppressive therapies[3]. No effective medical therapy for halting disease progression has been identified, but ursodeoxycholic acid is being assessed. A median duration of 12 to 18 years from the time of diagnosis before patients develop end-stage liver disease has been observed. Among eligible patients, liver transplantation (LT) is currently the only life-extending therapy for patients with end-stage PSC, although the disease can recur in the allografted liver and be a cause of morbidity post-transplant[4].
PSC affects primarily young and middle-aged men, especially patients with underlying inflammatory bowel disease. Approximately 70% to 80% of PSC patients in the United States have ulcerative colitis (UC)[5–10]. Conversely, approximately 2% to 7.5% of patients with UC[11] and 1.4% to 3.4% of patients with Crohn’s disease[12] develop PSC. IBD can be diagnosed at any time during the course of PSC, and PSC can occur at any time during the course of IBD[13]. In general, however, IBD is diagnosed several years earlier than PSC[13]. PSC may also develop many years after proctocolectomy for colitis and IBD can develop many years after liver transplantation due to advanced PSC[11]. Whether PSC is a distinct entity in patients with and without IBD might be a clinically significant issue[1415], but at present, there are not sufficient data to conclude that PSC occurring in patients without IBD is an entity separate from PSC found in association with IBD[1116].
Asymptomatic patients represent about 15% to 40% of the patients at time of diagnosis in early studies[2]. More recently, more patients are identified at an earlier stage of the disease with fewer symptoms. One study showed that the majority of patients (greater than 55%) initially present with asymptomatically elevated liver enzymes[17]. Due to its close association to IBD, many cases come to medical attention when patients with IBD are screened for liver disease.
The clinical course of PSC is typically one of insidious worsening of cholestasis and eventual development of jaundice and end-stage liver disease[3]. As such, asymptomatic patients with PSC are at increased risk for developing symptoms over time. Fatigue and pruritus are reported as the most common symptoms. Jaundice, pain, fever and weight loss, cholangiocarcinoma, or manifestations of portal hypertension in advanced stages of liver disease are uncommon initial manifestations. In one recent study, the most common presenting symptoms were described as abdominal pain (20%), pruritus (10%), diarrhea (8%), jaundice (6%), fatigue (6%) and fever (4%)[17]. Another recent study from Sweden suggested that more patients without IBD are identified and the patients are older at diagnosis[18]. Symptoms of bacterial cholangitis usually are not manifested until patients undergo endoscopic intervention or surgical exploration of the biliary tract[19]. Cholangiocarcinoma develops in up to 23% of patients[20] and can occur relatively early and before onset of cirrhosis[3].
Impairments in health-related quality of life among individuals with PSC compared to the general population were confirmed in two independent populations[2122]. Patients with cirrhosis form primary hepatocellular disease, however, reported lower health-related quality of life scores compared to patients with cholestatic liver disease[21].
A cholestatic picture of liver function with elevations in serum alkaline phosphatase values are the biochemical hallmark of PSC. Increases between 3 and 10 times the upper limit of normal occur in 95% of cases. Serum alanine and aspartate aminotransferase levels are usually 2-3 fold higher than normal levels. The serum total bilirubin level is normal in 60% of individuals at diagnosis[2]. The liver function tests, however, may be normal and can fluctuate during the course of the disease[23].
Several prognostic models for PSC have been developed, most of which include age, serum bilirubin and histologic staging[5791024]. Most recently, a Mayo model for predicting the survival has been refined[25]. This uses the age of the patient, total serum bilirubin, aspartate aminotransferase levels, presence or absence of variceal bleeding and serum albumin as independent variables, and can be used in early stages of PSC, before onset of cirrhosis. The limitations of prognostic models include the inability to account for the development of cholangiocarcinoma and health-related quality of life[19]. Once decompensated cirrhosis is present, the Model for End-Stage Liver Disease (MELD) score[26] more accurately predicts survival and is more appropriately used in prioritizing patients for liver transplantation[3].
Currently, testing for specific autoimmune antibodies does not contribute to the diagnosis of PSC. The prevalent autoantibody reactivity is a perinuclear antineutrophilic autoantibody (pANCA), present in approximately 80% of patients but lacking in diagnostic specificity[27–30]. The unidentified antigenic reactant is not the proteinase (myeloperoxidase) of conventional pANCA. Other autoantibodies such as antinuclear antibodies and smooth muscle antibodies occur in 20% to 60% of patients, usually in lower titers than those observed in autoimmune hepatitis[31]; their fine antigenic specificity has not been established. Antimitochondrial antibodies are rarely found in patients with PSC[6], in keeping with the lack of overlap between PSC and primary biliary cirrhosis (PBC). The serological markers of autoimmune liver diseases are covered in more detail in other articles in this series.
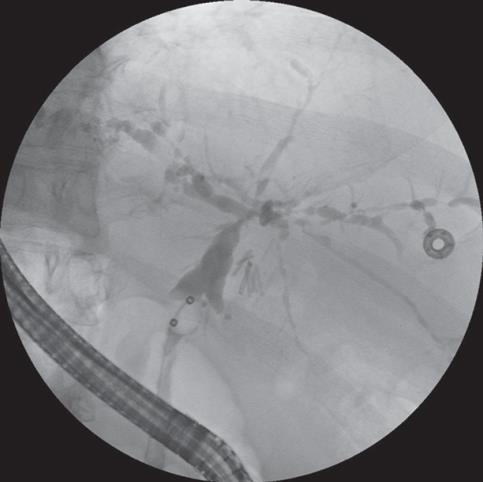
Cholangiography is considered to be the gold standard for the diagnosis of PSC[6]. In experienced hands, endoscopic retrograde cholangiopancreatography (ERCP) is successful in demonstrating the intra- and extra-hepatic biliary tree in 95% of the cases[3]. Segmental fibrosis of intrahepatic and/or extrahepatic bile ducts with saccular dilatation of normal intervening areas results in the characteristic beads-on-a-string appearance (Figure 1). Intrahepatic duct involvement is nearly universal with most patients affected by intrahepatic and extrahepatic disease[19]. Procedure-related complications from ERCP can occur in 3% to 8% of patients and include abdominal pain, pancreatitis, bleeding, common bile duct perforation, biliary sepsis and death[32–34].
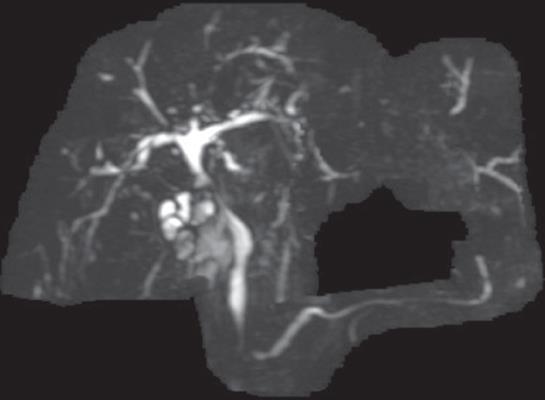
The use of magnetic resonance cholangiography (MRC) for detecting PSC has been evaluated as a rapid, noninvasive examination of the biliary tract (Figure 2). MRC has no significant morbidity when performed in appropriately selected individuals, and avoids the potential adverse effects of radiation exposure and contrast media associated with ERCP[35]. For the detection of PSC, MRC has been found to be accurate and comparable to ERCP[36–39]. Moreover, one study has suggested it also results in cost savings when used as the initial test strategy for diagnosing PSC[34]. Factors that lead to difficulties in interpreting the MRC compared to ERCP include the presence of cirrhosis and PSC limited to the peripheral intrahepatic bile ducts[36]. The major disadvantage of MRC is that it is a purely diagnostic examination, although it can be used to identify patients who would benefit from subsequent therapeutic ERCP[19]. Although biliary tree changes on MRC aid in the diagnosis of PSC, they do not correlate with survival, as predicted by the Mayo Risk Score[38].
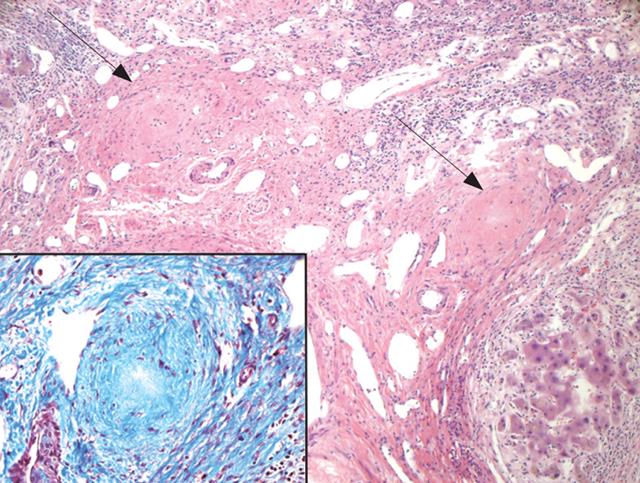
PSC is histologically characterized by damage, atrophy, and, ultimately, loss of medium- and large-sized bile ducts, within or outside the liver[4041]. These are not typically captured in a percutaneous liver biopsy. The histological picture is complicated by the fact that separation of the disease process itself from the effects of distal obstruction of bile ducts can be challenging[41]. The smaller ducts are affected by the resultant obstruction and gradually disappear (ductopenia). The characteristic pathologic features of PSC are concentric periductal fibrosis (“onion-skinning”) that progresses to a narrowing and then obliteration of the small bile ducts leaving a bile duct scar (Figure 3), but this is found in less than 15% of the patients with PSC[42]. Many of the biopsy changes, such as bile stasis, pseudoxanthomatous changes, Mallory bodies and copper accumulation, lack specificity for diagnostic purposes[41], and can occur with chronic extra-hepatic bile duct obstruction from any cause[43]. Several stages can be recognized histologically, ranging from stages I to IV: cholangitis and portal hepatitis (stageI); periportal fibrosis or periportal hepatitis (stage II); septal fibrosis, bridging necrosis or both (stage III); and biliary cirrhosis (stage IV)[44]. Sampling error is a significant limitation of liver biopsy[41].
Liver biopsy in patients with radiographic evidence of PSC is not needed for diagnosis, although it may help in excluding other diseases[45]. Histological staging may be complementary to ERCP evaluation, but most times is not necessary. Liver biopsy should probably be limited to patients with a challenging presentation or those being investigated for small duct PSC or possible overlap syndrome with autoimmune hepatitis[17]. The presence of biliary dysplasia on liver histology has been proposed as a marker for eventual cholangiocarcinoma in PSC[4647], but its use in clinical practice is limited by poor reproducibility of findings on pathologic interpretation[47].
Small duct PSC refers to disease that affects bile ducts that are too small to be identified by ERCP. This entity is characterized by a consistent liver histology and radiographically normal bile ducts[4849]. The proportion of small duct PSC to “large duct PSC” has been described as approximately 5%-15%[5051]. Small duct PSC is believed to be a distinct entity from the large duct form, with a less aggressive course and less likely to lead to cholangiocarcinoma[5052].
PSC with overlap features of autoimmune hepatitis has been reported both in the pediatric and adult populations of patients with PSC. In adults, the therapeutic response to immunosuppressants, in particular the autoimmune hepatitis- or hepatocellular component of the overlap syndrome, can be excellent, and can lead to complete remission of disease activity[53]. The response to therapy might be dependent on the predominance of AIH or PSC features. The overlap syndromes of autoimmune liver diseases, including overlap of AIH and PSC in childhood are covered in more detail in other articles in this series.
During the past decade, patients with steroid responsive sclerosing cholangitis have been described, often but not always associated with autoimmune pancreatitis[54]. Histological findings of lymphoplasmacytic infiltration and infiltration of IgG4-bearing plasma cells, and high serum IgG4 levels have been consistently observed and frequently required for diagnosis of autoimmune pancreatitis[5556], and have also been observed in the hepatobiliary system. Described hepatobiliary changes have included stenosis of the bile ducts, biliary duct wall thickening, stenosis of the portal vein, portal fibrosis[57], and hepatic inflammatory pseudotumor[58]. Hepatobiliary involvement without pancreatic involvement suggests that IgG4-related sclerosing cholangitis could be a distinct entity from autoimmune pancreatitis[58]. IgG4-related sclerosing cholangitis is similar to PSC with regard to cholangiographic features, but, in contrast to PSC, is susceptible to steroid therapy and is reversible[58]. Therefore, identifying patients with IgG4-related sclerosing cholangitis and distinguishing them from patients with PSC could have major therapeutic implications[59]. Although very limited data exists on the prognosis and natural history of IgG4-related sclerosing cholangitis, it seems that the prognosis of these patients is more favorable than that of patients with PSC[54].
Different forms of medical treatment have been tried, but until now, no treatment has been proven efficient in randomized controlled studies.
Several drugs, such as penicillamine[60], methotrexate[61], budesonide[62], colchicine[63], cladribine[64], cyclosporine[65], mycophenolate mofetil[6667], etanercept[68], oral and transdermal nicotine[6970], silymarin[71], pirfenidone[72], and pentoxifylline[73], have been evaluated in the treatment of this condition, but none of them has demonstrated convincing evidence of benefit and some are associated with significant side effects[6266].
Tacrolimus was shown in a pilot study to cause significant improvement in serum liver biochemistries including alkaline phosphatase[74]. A subsequent study from Mayo Clinic supports previous observations that oral tacrolimus is associated with significant reductions in alkaline phosphatase levels in PSC[75]. However, in this study, the drug was not well tolerated, and the clinical benefit with oral tacrolimus with respect to disease activity in PSC appears to be limited.
Biologic therapy has been a major advancement in the current therapy of inflammatory bowel disease. There are no controlled trials evaluating the role of biologic therapy (e.g. infliximab and adalimumab) in the management of PSC.
Multiple controlled studies have suggested that ursodeoxycholic acid (UDCA) has beneficial effects on liver biochemistries of patients with PSC[76–85]. A few studies have documented an improvement in liver histological appearance[767982]. Other studies have not included liver histology as an outcome mainly because of sampling issues[85]. However, UDCA has not yet proven to prolong survival or improve outcome of PSC. All the trials performed to date have been limited by small number of patients and relatively short follow-up periods.
In an open label study performed at Mayo Clinic, 30 patients with PSC received high-dose (25-30 mg/kg per day) UDCA[83], and substantial reduction not only in serum hepatic biochemistries but also Mayo risk score were observed after 12 mo of therapy. Differences in expected 4-year survival based on Mayo Risk Score were substantial between historical placebo and high-dose UDCA groups. A previous placebo-controlled study conducted at Mayo Clinic in which 51 patients received lower doses of UDCA (13-15 mg/kg per day)[77] had showed beneficial effects limited to serum hepatic biochemistries, but no difference in predicted survival. An independent, double-blind, placebo-controlled trial[82] of UDCA at 20 mg/kg per day involving 102 patients observed improvement in liver biochemistries, cholangiographic appearance and liver histology after 2 years of therapy, but failed to have any significant effect on survival. No significant UDCA related adverse events were reported from either study. On the other hand, a European study[85] with 219 patients who were randomized to receive either high-dose UDCA (17-23 mg/kg per day) or placebo for 5 years did not observe any significant decrease of serum alkaline phosphatase in the UDCA-treated patients. There was no significant benefit from UDCA on survival without liver transplantation or prevention of cholangiocarcinoma, but the study was too small to exclude a significant beneficial effect on survival. A large, multicenter National Institutes of Health sponsored randomized trial of high-dose UDCA is currently underway[86].
Combination therapy is a relatively new avenue of clinical research in the treatment of chronic cholestatic diseases. Drugs in monotherapy are often limited by efficacy and dose-related toxicity; combination therapy may hold the potential for improved efficacy through additive or synergistic effects, with the potential minimization of drug toxicities[87]. A controlled but nonrandomized study with 12 patients treated with a combination of low-dose prednisolone and colchicine failed to find any benefit in PSC[88]. The combination of UDCA and methotrexate was studied in 19 patients with PSC and no changes in biochemistries from baseline values were seen compared to patients receiving UDCA alone[89]. An 8-wk pilot study evaluating the combination of prednisone or budesonide combined with UDCA failed to demonstrate significant beneficial effects to justify its use in patients with PSC[90]. In a small study with 15 patients, positive results were obtained with the combination of UDCA, prednisolone and azathioprine in decreasing liver biochemistry values[91], but evidence supporting long-term use of this therapy is lacking. Most recently, a study with 80 PSC patients randomized to either UDCA alone or the combination of metronidazole and UDCA showed that combination therapy led to improved serum alkaline phosphatase and Mayo Risk Score, but no significant effect on disease progression compared to UDCA alone[92].
Trials of antibiotics such as metronidazole and minocycline have been promising but inconclusive. A small study of docosahexaenoic acid (DHA) which improves CFTR function[93] is currently underway. Most promising for the near future are inhibitors of TNF action, antifibrotic agents (such as angiotensin-converting enzyme [ACE] inhibitors, sirolimus/rapamycin), and inhibitors of formation of toxic bile (such as 24-norursodeoxycholic acid)[94].
Some patients present with clinical and biochemical deterioration and exhibit a dominant stricture that involves the larger extrahepatic biliary ducts. Such lesions may be amenable to endoscopic or radiologic dilatation with or without a biliary drainage procedure, such as sphincterotomy and stenting[45]. This leads to improvement of clinical symptoms, liver biochemistries and cholangiographic findings. However, the endoscopic treatment of PSC has generated controversy, not only with regard to optimal management, but also its overall influence on survival. The use of endobiliary stents has been compared to balloon dilatation alone in patients with PSC[9596], and a greater frequency of intervention-related complications including acute cholangitis was observed in patients with endobiliary stent placement. Repeated balloon dilatations of dominant biliary strictures resulted in improved actual survival rates compared to survival rates predicted by Mayo risk score[9798].
Dominant strictures can also be managed surgically by dilatation or choledochojejunostomy, but this treatment has become uncommon with more recent advancement of endoscopic techniques and growing success of liver transplantation. At present, biliary surgery in patients with PSC, other than simple cholecystectomy, should be minimized and reserved for the selected rare noncirrhotic patients who have marked cholestasis or recurrent cholangitis caused by a dominant extrahepatic or hilar stricture not amenable to endoscopic or percutaneous dilatation[45]. In patients who may undergo liver transplantation, prior biliary surgery has been associated with a significantly longer operation time, greater intraoperative blood loss, and a higher incidence of biliary complications post-liver transplantation compared with those patients with no history of biliary surgery[99–103].
Although PSC is an uncommon disease, advanced-stage PSC remains among the most common indications for LT in the United States and in Europe[20]. Unique circumstances that require evaluation for possible LT include recurrent bacterial cholangitis despite intensive medical and endoscopic therapy, severe extrahepatic biliary obstruction that precludes operative repair, and uncontrolled peristomal variceal bleeding. Intractable pruritus may also be an indication for liver transplantation. Liver transplantation should be considered before the disease is too advanced, in order to enhance the long-term survival rates post-liver transplantation[104]. Prognostic models can aid in the timing of liver transplantation. Reports from single centers performing LT in PSC patients have demonstrated excellent survival rates of 90%-97% at one year, and 83%-88% at 5 years[105106]. However, retransplantation rates seem to be higher for patients with PSC than other diagnoses[3].
Recurrence of PSC in the liver graft has been documented. Diagnosis of recurrence can be challenging, as non-specific bile duct injuries and strictures caused by allograft reperfusion injury, ischemia, rejection and recurrent biliary sepsis can mimic the findings of PSC post-transplantation and need to be carefully excluded before the diagnosis of recurrence can be established[107108]. The frequency of recurrent PSC after liver transplantation remains controversial. The frequency of recurrent disease is estimated between 10% to 20% of patients[109], but a recent systematic review has indicated that publication bias might be a concern regarding this topic[4]. PSC might recur earlier at a higher ratio after living donor liver transplantation, particularly when the liver graft is obtained from a biologically related living donor[110]. Proposed risk factors for recurrent PSC include inflammatory bowel disease, prolonged cold ischemia time, number of cellular rejection episodes, previous biliary surgery, cytomegalovirus infection, and lymphocytotoxic cross-match[4] but these require further investigation. As more liver transplant recipients survive longer, the recurrence of disease may become the primary cause of morbidity and mortality in PSC[4].
Liver transplantation in autoimmune liver diseases is covered in more detail in another article in this series.
Pruritus is a prevalent problem in patients with chronic cholestatic disease[111]. Cholestyramine is effective in 80% to 90% of the patients, and represents the first-line of treatment[112]. Other drugs that have been commonly used for the treatment of pruritus include rifampin[113], opioid antagonists[114115] and ondansetron[116]. Sertraline has been demonstrated to have positive effects on pruritus in cholestatic liver disease in a small study[117]. Etanercept has also had positive effects on pruritus in patients with PSC in a small study not originally designed to primarily assess the effect of that drug on pruritus[68]. In controlled trials, UDCA has not been associated with improvement in pruritus, but those studies were not specifically designed for that purpose. Intractable pruritus may be an indication for LT[19].
Fatigue is also noted to be a prevalent problem in patients with chronic cholestatic disease, and a major determinant of impaired health related quality of life[118]. However, no medical therapy is available for treatment.
Severe bone disease in PSC patients is more common than expected, but less frequent than that reported in primary biliary cirrhosis[119]. Patients with longer duration of IBD, and more advanced liver disease were found to be at higher risk of severe osteoporosis[119]. In this same study, Angulo et al[119] found that the severity of osteopenic disease in PSC seems to increase as liver disease advances, but this finding was not confirmed in a subsequent study by Campbell et al[120]. Bone mineral densitometry measurements are the only test helpful in evaluating progression of osteopenia in patients with PSC, and the presence, severity and progression of the bone disease cannot be accurately evaluated by routine clinical, biochemical, or histological variables[119]. This is important since most patients with PSC and advanced liver disease undergo liver transplantation. Early post-transplant bone loss remains a clinically significant problem and frequently leads to fracturing in a third of patients with PSC when the pre-transplant bone mineral density is below the fracture threshold[119121].
For treatment of osteoporosis and osteopenia, calcium and vitamin D supplementation are recommended, and in selected cases, bisphosphonates may be indicated. Many new drugs have become available for the treatment of post-menopausal osteoporosis, and more studies are needed to determine the role of these treatments in primary sclerosing cholangitis.
Cholelithiasis has been noted in 26% of individuals with PSC, with the majority being asymptomatic[122]. Consideration should be given to performing a cholecystectomy if cross-sectional imaging results in the identification of gallbladder polyps given the potential for neoplastic transformation in PSC[123].
A special complication of portal hypertension in PSC patients with an ileal stoma is the development of peristomal varices[45]. Those develop within the adhesions between the ileal (portal) veins and the anterior abdominal wall (systemic) veins. Patients bleeding from peristomal varices often present with recurrent hemorrhagic episodes. Bleeding from the peristomal varices is more difficult to treat than bleeding from esophageal varices. Local treatment to control and prevent bleeding is usually unsuccessful in the long term. Liver transplantation should be considered for treatment. If that is not possible, peristomal variceal bleeding can be controlled with a portosystemic shunt[2].
A dominant stricture, defined by Stiehl et al[124] as a diameter in the common duct of less than 1.5 mm and in the hepatic duct of less than 1 mm, is a frequent finding and occurs in 45% to 58% of patients during follow-up. Stenotic lesions in PSC are thus far more often benign than malignant in nature[124]. Endoscopic treatment of dominant stenoses improves cholestasis and prolongs survival in comparison to predicted survival[97125]. Prophylactic antibiotic administration prior to endoscopic manipulation of the biliary tree is recommended by the American Society for Gastrointestinal Endoscopy in the setting of bile duct obstruction to prevent contamination during the cannulation of the bile duct[126].
Bacteriobilia is found in the majority of PSC patients[127], but, as previously mentioned, bacterial cholangitis usually is not manifested until patients undergo endoscopic intervention or surgical exploration of the biliary tract. Bacterial cholangitis is common in patients with dominant stricture and requires antibiotic treatment[45]. It may also occur after endoscopic procedures or in patients with bile duct stones or tight strictures[128], warranting prophylactic antibiotic administration prior to endoscopic manipulation of the biliary tree. Most biliary infections in patients with obstructive disease of the biliary tract are caused by aerobic enteric organisms such as Escheria coli, Klebsiella species, and E. faecalis[129]. Recurrent episodes of bacterial cholangitis can be an indication for liver transplantation in patients with otherwise preserved liver function. Prophylaxis with antibiotics has not been proven to be of benefit[128], but patients with recurrent cholangitis should be advised to seek medical attention rapidly and start antibiotics at the first sign of biliary infection.
Cholangiocarcinoma: Primary sclerosing cholangitis carries an increased risk of hepatobiliary malignancy, especially cholangiocarcinoma (CCA)[8130]. The development of CCA is the most lethal complication of PSC. Cholangiocarcinoma can arise at any stage of PSC, although, in general, the incidence is higher in more advanced disease[5131]. There are no clinical features that predict the diagnosis of CCA, and diagnosis can be challenging.
The cumulative life-time incidence of CCA is estimated as 6% to 23%[20]. The reported prevalence of CCA in explanted livers and autopsy is much higher, approximately 30% to 42%[9132]. Overall, up to 50% of CCA cases are detected synchronous with the PSC diagnosis or within one year of diagnosis of PSC[130133–136]. Based upon the same reported series, the incidence of CCA during follow-up, starting at 1 year after the diagnosis of PSC, can be calculated as being between 0.5% and 1.5% per year. The malignancy usually develops in the fourth decade of life, whereas CCA in patients without PSC usually develops much later in life, in their seventh decade of life[20].
Risk factors for the development of CCA in PSC have not been clearly identified, but older age, longer duration of IBD and smoking behavior have been associated with an increased risk for development of CCA in patients with PSC. Finding biliary dysplasia on liver histology has also been proposed as a precursor in the development of cholangiocarcinoma[4647].
The diagnosis of CCA can be challenging. The role of serum CA19-9 level in the diagnosis of CCA is controversial. There are no tumor markers which are specific for cholangiocarcinoma. In the context of PSC, a serum CA19-9 level greater than 100 U/mL has been reported to have a sensitivity of 75% and a specificity of 80% for presence of cholangiocarcinoma[137138]. A recent study from the Mayo Clinic found that a serum level greater than 129 U/mL provided a sensitivity of 78.6% and specificity of 98.5% for CCA in PSC[139]. Even though these studies suggest CA 19-9 is an accurate test to diagnose cholangiocarcinoma, CA 19-9 was only found to identify patients with advanced, unresectable CCA, and thus its use is not appropriate as a screening test[139]. Ultrasonography, computed tomography, and magnetic resonance have inadequate sensitivity to distinguish CCA from PSC. Endoscopic biopsy and biliary brushing for cytology, digital image analysis, and fluorescent in situ hybridization are noted for good specificity but poor sensitivity in detecting CCA[19].
Patients with PSC and CCA have a very poor outcome, with median survival of approximately 5 to 11 mo[20135140]. Even though survival of patients in whom CCA was found incidentally by histological examination of the explanted liver has been reported to be good[5], in general, LT for patients with CCA results in a low success rate[141–144]. However, more recent data from investigational protocols have suggested better outcomes in highly selected individuals. The use of pretreatment radiotherapy and subsequent capecitabine for 2 to 3 wk prior to LT at Mayo Clinic has yielded a 3- and 5-year actuarial survival of 82%[145]. The use of brachytherapy and continuous 5-fluoracil infusion before liver transplantation in Nebraska resulted in 45% long-term cancer-free survival after follow-up for a median of 7.5 years[146]. Curative resection among individuals with early-stage cholangiocarcinoma may also be of benefit in PSC[133], although recent data suggest that transplant with neoadjuvant chemoradiation with localized, node-negative hilar CCA may achieve better survival with less recurrence than conventional resection[145147].
Recent studies have suggested that the incidence of CCA in patients with PSC treated with UDCA is lower than expected and decreases with time of therapy[141148]. Further studies are needed to confirm this finding.
Colonic dysplasia and carcinoma: Whether the presence of PSC increases the risk of colonic dysplasia and carcinoma in ulcerative colitis is controversial[149150]. Patients with PSC and UC have been found to have an increased incidence of colonic carcinomas compared to patients with ulcerative colitis alone in a few studies[136151–153], however, contradictory results have also been presented[154155]. The size, design, end-points, and populations involved in these studies have varied, and critical review suggests that colorectal cancer is more common in the setting of PSC[150]. Furthermore, PSC patients with UC remain at an increased risk for developing colorectal dysplasia and carcinoma after they have undergone liver transplantation[11156]. The immunosuppressive treatment after liver transplantation may have an impact on the development of cancer.
Two studies have indicated that UDCA reduced the incidence of colonic dysplasias and/or carcinomas[157158].
Gallbladder neoplasia: Dysplasia, adenomas and carcinoma of the gallbladder have been described in PSC but are less common than cholangiocarcinoma. PSC is recognized as one of the major risk factors for both gallbladder and bile duct carcinoma[159]. A recent study reported statistically significant association between hilar/intrahepatic biliary neoplasia and gallbladder neoplasia, suggesting a “field effect” in the intrahepatic and extrahepatic biliary tree in PSC[160]. Identification of gallbladder polyps on cross-sectional imaging should lead to consideration for cholecystectomy[123]. Large studies on this subject have not been performed.
Hepatocellular carcinoma: Although patients with cirrhotic stage PSC may also be at risk for developing hepatocellular carcinoma, this malignancy occurs infrequently[131136161].
Little is known regarding the natural history and potential complications of pregnancy in patients with PSC. There are very few case reports[162163] and one small series of thirteen pregnancies in 10 patients with PSC[164] that describe the fetal and maternal outcome of pregnancy in PSC. Although previously described, it appears that hepatic disease activity is not significantly worsened during the gestational period[2]. Nonetheless, patients with PSC who become pregnant require close monitoring[162]. Regular blood tests, including serum bilirubin and aminotransferase levels, are essential. In the event that the patient develops symptoms worrisome for obstruction, an ultrasound is a safe diagnostic test and may detect the presence of dominant strictures or stones; it lacks sensitivity however. MRC might have an emerging role in pregnancy, and more invasive tests such as ERCP might be required.
Primary sclerosing cholangitis is a presumed immune-mediated liver disease of young men associated with significant morbidity and mortality. However, there is no proven medical treatment available for it. Further studies are needed for better understanding of the pathophysiology of the disease and for development of an optimal therapeutic strategy for patients with PSC to improve health related quality of life and halt progression of disease, thereby decreasing incidence of complications of advanced liver disease, and the need for transplantation.
| 1. | Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332:924-933. |
| 2. | Talwalkar JA, Lindor KD. Primary sclerosing cholangitis. Inflamm Bowel Dis. 2005;11:62-72. |
| 3. | LaRusso NF, Shneider BL, Black D, Gores GJ, James SP, Doo E, Hoofnagle JH. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746-764. |
| 4. | Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813-1824. |
| 5. | Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717. |
| 6. | Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870-877. |
| 7. | Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, Fleming TR, Fisher LD, Beaver SJ, LaRusso NF. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430-436. |
| 8. | Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200-206. |
| 9. | Broome U, Olsson R, Loof L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzen H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. |
| 10. | Okolicsanyi L, Fabris L, Viaggi S, Carulli N, Podda M, Ricci G. Primary sclerosing cholangitis: clinical presentation, natural history and prognostic variables: an Italian multicentre study. The Italian PSC Study Group. Eur J Gastroenterol Hepatol. 1996;8:685-691. |
| 11. | Broome U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. |
| 12. | Rasmussen HH, Fallingborg JF, Mortensen PB, Vyberg M, Tage-Jensen U, Rasmussen SN. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn's disease. Scand J Gastroenterol. 1997;32:604-610. |
| 13. | Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31-39. |
| 14. | Rabinovitz M, Gavaler JS, Schade RR, Dindzans VJ, Chien MC, Van Thiel DH. Does primary sclerosing cholangitis occurring in association with inflammatory bowel disease differ from that occurring in the absence of inflammatory bowel disease? A study of sixty-six subjects. Hepatology. 1990;11:7-11. |
| 15. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. |
| 16. | Saarinen S, Olerup O, Broome U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:3195-3199. |
| 17. | Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102:1042-1049. |
| 18. | Bergquist A, Said K, Broome U. Changes over a 20-year period in the clinical presentation of primary sclerosing cholangitis in Sweden. Scand J Gastroenterol. 2007;42:88-93. |
| 19. | Charatcharoenwitthaya P, Lindor KD. Primary sclerosing cholangitis: diagnosis and management. Curr Gastroenterol Rep. 2006;8:75-82. |
| 20. | Bjornsson E, Angulo P. Cholangiocarcinoma in young individuals with and without primary sclerosing cholangitis. Am J Gastroenterol. 2007;102:1677-1682. |
| 21. | Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96:2199-2205. |
| 22. | Kim WR, Lindor KD, Malinchoc M, Petz JL, Jorgensen R, Dickson ER. Reliability and validity of the NIDDK-QA instrument in the assessment of quality of life in ambulatory patients with cholestatic liver disease. Hepatology. 2000;32:924-929. |
| 23. | Cullen SN, Chapman RW. Review article: current management of primary sclerosing cholangitis. Aliment Pharmacol Ther. 2005;21:933-948. |
| 24. | Dickson ER, Murtaugh PA, Wiesner RH, Grambsch PM, Fleming TR, Ludwig J, LaRusso NF, Malinchoc M, Chapman RW, Kaplan MM. Primary sclerosing cholangitis: refinement and validation of survival models. Gastroenterology. 1992;103:1893-1901. |
| 25. | Kim WR, Therneau TM, Wiesner RH, Poterucha JJ, Benson JT, Malinchoc M, LaRusso NF, Lindor KD, Dickson ER. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688-694. |
| 26. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. |
| 27. | Chapman RW, Cottone M, Selby WS, Shepherd HA, Sherlock S, Jewell DP. Serum autoantibodies, ulcerative colitis and primary sclerosing cholangitis. Gut. 1986;27:86-91. |
| 28. | Mulder AH, Horst G, Haagsma EB, Limburg PC, Kleibeuker JH, Kallenberg CG. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411-417. |
| 29. | Bansi D, Chapman R, Fleming K. Antineutrophil cytoplasmic antibodies in chronic liver diseases: prevalence, titre, specificity and IgG subclass. J Hepatol. 1996;24:581-586. |
| 30. | Chapman RW. The enigma of anti-neutrophil antibodies in ulcerative colitis primary sclerosing cholangitis: important genetic marker or epiphenomenon? Hepatology. 1995;21:1473-1474. |
| 31. | Wiesner RH. Current concepts in primary sclerosing cholangitis. Mayo Clin Proc. 1994;69:969-982. |
| 32. | Bilbao MK, Dotter CT, Lee TG, Katon RM. Complications of endoscopic retrograde cholangiopancreatography (ERCP). A study of 10,000 cases. Gastroenterology. 1976;70:314-320. |
| 33. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. |
| 34. | Talwalkar JA, Angulo P, Johnson CD, Petersen BT, Lindor KD. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology. 2004;40:39-45. |
| 35. | Mehta SN, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography. Gastrointest Endosc Clin N Am. 1997;7:247-270. |
| 36. | Fulcher AS, Turner MA, Franklin KJ, Shiffman ML, Sterling RK, Luketic VA, Sanyal AJ. Primary sclerosing cholangitis: evaluation with MR cholangiography-a case-control study. Radiology. 2000;215:71-80. |
| 37. | Moff SL, Kamel IR, Eustace J, Lawler LP, Kantsevoy S, Kalloo AN, Thuluvath PJ. Diagnosis of primary sclerosing cholangitis: a blinded comparative study using magnetic resonance cholangiography and endoscopic retrograde cholangiography. Gastrointest Endosc. 2006;64:219-223. |
| 38. | Petrovic BD, Nikolaidis P, Hammond NA, Martin JA, Petrovic PV, Desai PM, Miller FH. Correlation Between Findings on MRCP and Gadolinium-Enhanced MR of the Liver and a Survival Model for Primary Sclerosing Cholangitis. Dig Dis Sci. 2007;52:3499-3506. |
| 39. | Angulo P, Pearce DH, Johnson CD, Henry JJ, LaRusso NF, Petersen BT, Lindor KD. Magnetic resonance cholangiography in patients with biliary disease: its role in primary sclerosing cholangitis. J Hepatol. 2000;33:520-527. |
| 40. | Ludwig J. Surgical pathology of the syndrome of primary sclerosing cholangitis. Am J Surg Pathol. 1989;13 Suppl 1:43-49. |
| 41. | Scheuer PJ. Ludwig Symposium on biliary disorders--part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc. 1998;73:179-183. |
| 42. | Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis? Am J Gastroenterol. 2003;98:1155-1158. |
| 43. | Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005;100:1330-1333. |
| 44. | Ludwig J, Barham SS, LaRusso NF, Elveback LR, Wiesner RH, McCall JT. Morphologic features of chronic hepatitis associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hepatology. 1981;1:632-640. |
| 45. | Angulo P, Lindor KD. Primary sclerosing cholangitis. Hepatology. 1999;30:325-332. |
| 46. | Bergquist A, Glaumann H, Stal P, Wang GS, Broome U. Biliary dysplasia, cell proliferation and nuclear DNA-fragmentation in primary sclerosing cholangitis with and without cholangiocarcinoma. J Intern Med. 2001;249:69-75. |
| 47. | Fleming KA, Boberg KM, Glaumann H, Bergquist A, Smith D, Clausen OP. Biliary dysplasia as a marker of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2001;34:360-365. |
| 48. | Bjornsson E, Chapman RW. Sclerosing cholangitis. Curr Opin Gastroenterol. 2003;19:270-275. |
| 49. | Kim WR, Ludwig J, Lindor KD. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol. 2000;95:1130-1138. |
| 50. | Bjornsson E, Boberg KM, Cullen S, Fleming K, Clausen OP, Fausa O, Schrumpf E, Chapman RW. Patients with small duct primary sclerosing cholangitis have a favourable long term prognosis. Gut. 2002;51:731-735. |
| 51. | Boberg KM, Schrumpf E, Fausa O, Elgjo K, Kolmannskog F, Haaland T, Holter E. Hepatobiliary disease in ulcerative colitis. An analysis of 18 patients with hepatobiliary lesions classified as small-duct primary sclerosing cholangitis. Scand J Gastroenterol. 1994;29:744-752. |
| 52. | Angulo P, Maor-Kendler Y, Lindor KD. Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology. 2002;35:1494-1500. |
| 53. | van Buuren HR, van Hoogstraten HJE, Terkivatan T, Schalm SW, Vleggaar FP. High prevalence of autoimmune hepatitis among patients with primary sclerosing cholangitis. J Hepatol. 2000;33:543-548. |
| 54. | Bjornsson E, Chari ST, Smyrk TC, Lindor K. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547-1554. |
| 55. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. |
| 56. | Okazaki K, Uchida K, Chiba T. Recent concept of autoimmune-related pancreatitis. J Gastroenterol. 2001;36:293-302. |
| 57. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Extrapancreatic lesions in autoimmune pancreatitis. J Clin Gastroenterol. 2005;39:904-907. |
| 58. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193-1203. |
| 59. | Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, Lindor KD. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070-2075. |
| 60. | LaRusso NF, Wiesner RH, Ludwig J, MacCarty RL, Beaver SJ, Zinsmeister AR. Prospective trial of penicillamine in primary sclerosing cholangitis. Gastroenterology. 1988;95:1036-1042. |
| 61. | Knox TA, Kaplan MM. A double-blind controlled trial of oral-pulse methotrexate therapy in the treatment of primary sclerosing cholangitis. Gastroenterology. 1994;106:494-499. |
| 62. | Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2333-2337. |
| 63. | Olsson R, Broome U, Danielsson A, Hagerstrand I, Jarnerot G, Loof L, Prytz H, Ryden BO, Wallerstedt S. Colchicine treatment of primary sclerosing cholangitis. Gastroenterology. 1995;108:1199-1203. |
| 64. | Duchini A, Younossi ZM, Saven A, Bordin GM, Knowles HJ, Pockros PJ. An open-label pilot trial of cladibrine (2-cholordeoxyadenosine) in patients with primary sclerosing cholangitis. J Clin Gastroenterol. 2000;31:292-296. |
| 65. | Sandborn WJ, Wiesner RH, Tremaine WJ, Larusso NF. Ulcerative colitis disease activity following treatment of associated primary sclerosing cholangitis with cyclosporin. Gut. 1993;34:242-246. |
| 66. | Talwalkar JA, Angulo P, Keach JC, Petz JL, Jorgensen RA, Lindor KD. Mycophenolate mofetil for the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2005;100:308-312. |
| 67. | Sterling RK, Salvatori JJ, Luketic VA, Sanyal AJ, Fulcher AS, Stravitz RT, Contos MJ, Mills AS, Shiffman ML. A prospective, randomized-controlled pilot study of ursodeoxycholic acid combined with mycophenolate mofetil in the treatment of primary sclerosing cholangitis. Aliment Pharmacol Ther. 2004;20:943-949. |
| 68. | Epstein MP, Kaplan MM. A pilot study of etanercept in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2004;49:1-4. |
| 69. | Angulo P, Bharucha AE, Jorgensen RA, DeSotel CK, Sandborn WJ, Larusso NF, Lindor KD. Oral nicotine in treatment of primary sclerosing cholangitis: a pilot study. Dig Dis Sci. 1999;44:602-607. |
| 70. | Vleggaar FP, van Buuren HR, van Berge Henegouwen GP, Hop WC, van Erpecum KJ. No beneficial effects of transdermal nicotine in patients with primary sclerosing cholangitis: results of a randomized double-blind placebo-controlled cross-over study. Eur J Gastroenterol Hepatol. 2001;13:171-175. |
| 71. | Angulo P. Silymarin in the treatment of primary sclerosing cholangitis: a pilot study. Gastroenterology. 2001;120:A353. |
| 72. | Angulo P, MacCarty RL, Sylvestre PB, Jorgensen RA, Wiesner RH, LaRusso NA, Lindor KD. Pirfenidone in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2002;47:157-61. |
| 73. | Bharucha AE, Jorgensen R, Lichtman SN, LaRusso NF, Lindor KD. A pilot study of pentoxifylline for the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2338-2342. |
| 74. | Van Thiel DH, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, Irish W, McMichael J, Starzl TE. Tacrolimus (FK 506), a treatment for primary sclerosing cholangitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:455-459. |
| 75. | Talwalkar JA, Gossard AA, Keach JC, Jorgensen RA, Petz JL, Lindor RN. Tacrolimus for the treatment of primary sclerosing cholangitis. Liver Int. 2007;27:451-453. |
| 76. | Beuers U, Spengler U, Kruis W, Aydemir U, Wiebecke B, Heldwein W, Weinzierl M, Pape GR, Sauerbruch T, Paumgartner G. Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology. 1992;16:707-714. |
| 77. | Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691-695. |
| 78. | O'Brien CB, Senior JR, Arora-Mirchandani R, Batta AK, Salen G. Ursodeoxycholic acid for the treatment of primary sclerosing cholangitis: a 30-month pilot study. Hepatology. 1991;14:838-847. |
| 79. | Stiehl A, Walker S, Stiehl L, Rudolph G, Hofmann WJ, Theilmann L. Effect of ursodeoxycholic acid on liver and bile duct disease in primary sclerosing cholangitis. A 3-year pilot study with a placebo-controlled study period. J Hepatol. 1994;20:57-64. |
| 80. | De Maria N, Colantoni A, Rosenbloom E, Van Thiel DH. Ursodeoxycholic acid does not improve the clinical course of primary sclerosing cholangitis over a 2-year period. Hepatogastroenterology. 1996;43:1472-1479. |
| 81. | van Hoogstraten HJ, Wolfhagen FH, van de Meeberg PC, Kuiper H, Nix GA, Becx MC, Hoek AC, van Houte DP, Rijk MC, Salemans JM. Ursodeoxycholic acid therapy for primary sclerosing cholangitis: results of a 2-year randomized controlled trial to evaluate single versus multiple daily doses. J Hepatol. 1998;29:417-423. |
| 82. | Mitchell SA, Bansi DS, Hunt N, Von Bergmann K, Fleming KA, Chapman RW. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121:900-907. |
| 83. | Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558-1562. |
| 84. | Okolicsanyi L, Groppo M, Floreani A, Morselli-Labate AM, Rusticali AG, Battocchia A, Colombo M, Galatola G, Gasbarrini G, Podda M. Treatment of primary sclerosing cholangitis with low-dose ursodeoxycholic acid: results of a retrospective Italian multicentre survey. Dig Liver Dis. 2003;35:325-331. |
| 85. | Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, Bell H, Gangsoy-Kristiansen M, Matre J, Rydning A. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464-1472. |
| 86. | Hoofnagle JH. Primary sclerosing cholangitis. Hepatology. 2005;41:955. |
| 87. | Fong DG, Lindor KD. Future directions in the medical treatment of primary sclerosing cholangitis: the need for combination drug therapy. Am J Gastroenterol. 2000;95:1861-1862. |
| 88. | Lindor KD, Wiesner RH, Colwell LJ, Steiner B, Beaver S, LaRusso NF. The combination of prednisone and colchicine in patients with primary sclerosing cholangitis. Am J Gastroenterol. 1991;86:57-61. |
| 89. | Lindor KD, Jorgensen RA, Anderson ML, Gores GJ, Hofmann AF, LaRusso NF. Ursodeoxycholic acid and methotrexate for primary sclerosing cholangitis: a pilot study. Am J Gastroenterol. 1996;91:511-515. |
| 90. | van Hoogstraten HJ, Vleggaar FP, Boland GJ, van Steenbergen W, Griffioen P, Hop WC, van Hattum J, van Berge Henegouwen GP, Schalm SW, van Buuren HR. Budesonide or prednisone in combination with urso-deoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am J Gastroenterol. 2000;95:2015-2022. |
| 91. | Schramm C, Schirmacher P, Helmreich-Becker I, Gerken G, zum Buschenfelde KH, Lohse AW. Combined therapy with azathioprine, prednisolone, and ursodiol in patients with primary sclerosing cholangitis. A case series. Ann Intern Med. 1999;131:943-946. |
| 92. | Farkkila M, Karvonen AL, Nurmi H, Nuutinen H, Taavitsainen M, Pikkarainen P, Karkkainen P. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379-1386. |
| 93. | Pall H, Zaman MM, Andersson C, Freedman SD. Decreased peroxisome proliferator activated receptor alpha is associated with bile duct injury in cystic fibrosis transmembrane conductance regulator-/- mice. J Pediatr Gastroenterol Nutr. 2006;42:275-281. |
| 94. | Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465-481. |
| 95. | Kaya M, Petersen BT, Angulo P, Baron TH, Andrews JC, Gostout CJ, Lindor KD. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059-1066. |
| 96. | Linder S, Soderlund C. Endoscopic therapy in primary sclerosing cholangitis: outcome of treatment and risk of cancer. Hepatogastroenterology. 2001;48:387-392. |
| 97. | Stiehl A, Rudolph G, Sauer P, Benz C, Stremmel W, Walker S, Theilmann L. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8-year prospective study. J Hepatol. 1997;26:560-566. |
| 98. | Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308-312. |
| 99. | McEntee G, Wiesner RH, Rosen C, Cooper J, Wahlstrom E. A comparative study of patients undergoing liver transplantation for primary sclerosing cholangitis and primary biliary cirrhosis. Transplant Proc. 1991;23:1563-1564. |
| 100. | Muiesan P, Shanmugam RP, Devlin J, Rela M, Heaton ND, Saxena R, Portmann B, Tan KC, Williams R. Orthotopic liver transplantation for primary sclerosing cholangitis. Transplant Proc. 1994;26:3574-3576. |
| 101. | Farges O, Malassagne B, Sebagh M, Bismuth H. Primary sclerosing cholangitis: liver transplantation or biliary surgery. Surgery. 1995;117:146-155. |
| 102. | Narumi S, Roberts JP, Emond JC, Lake J, Ascher NL. Liver transplantation for sclerosing cholangitis. Hepatology. 1995;22:451-457. |
| 103. | Ahrendt SA, Pitt HA, Kalloo AN, Venbrux AC, Klein AS, Herlong HF, Coleman J, Lillemoe KD, Cameron JL. Primary sclerosing cholangitis: resect, dilate, or transplant? Ann Surg. 1998;227:412-423. |
| 104. | Nashan B, Schlitt HJ, Tusch G, Oldhafer KJ, Ringe B, Wagner S, Pichlmayr R. Biliary malignancies in primary sclerosing cholangitis: timing for liver transplantation. Hepatology. 1996;23:1105-1111. |
| 105. | Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886-897. |
| 106. | Merion RM. When is a patient too well and when is a patient too sick for a liver transplant? Liver Transpl. 2004;10:S69-S73. |
| 107. | Khettry U, Keaveny A, Goldar-Najafi A, Lewis WD, Pomfret EA, Pomposelli JJ, Jenkins RL, Gordon FD. Liver transplantation for primary sclerosing cholangitis: a long-term clinicopathologic study. Hum Pathol. 2003;34:1127-1136. |
| 108. | Brandsaeter B, Schrumpf E, Clausen OP, Abildgaard A, Hafsahl G, Bjoro K. Recurrent sclerosing cholangitis or ischemic bile duct lesions--a diagnostic challenge? Liver Transpl. 2004;10:1073-1074. |
| 109. | Gordon F. Recurrent primary sclerosing cholangitis: Clinical diagnosis and long-term management issues. Liver Transpl. 2006;12:S73-S75. |
| 110. | Tamura S, Sugawara Y, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Recurrence of primary sclerosing cholangitis after living donor liver transplantation. Liver Int. 2007;27:86-94. |
| 111. | Jones EA, Bergasa NV. The pruritus of cholestasis. Hepatology. 1999;29:1003-1006. |
| 112. | Polter DE, Gruhl V, Eigenbrodt EH, Combes B. Beneficial effect of cholestyramine in sclerosing cholangitis. Gastroenterology. 1980;79:326-333. |
| 113. | Tabibian N. Rifampin as antipruritic agent in primary sclerosing cholangitis. Am J Gastroenterol. 1989;84:340. |
| 114. | Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113:1264-1269. |
| 115. | Bergasa NV, Alling DW, Talbot TL, Swain MG, Yurdaydin C, Turner ML, Schmitt JM, Walker EC, Jones EA. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med. 1995;123:161-167. |
| 116. | Jones EA, Molenaar HA, Oosting J. Ondansetron and pruritus in chronic liver disease: a controlled study. Hepatogastroenterology. 2007;54:1196-1199. |
| 117. | Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666-674. |
| 118. | Poupon RE, Chretien Y, Chazouilleres O, Poupon R, Chwalow J. Quality of life in patients with primary biliary cirrhosis. Hepatology. 2004;40:489-494. |
| 119. | Angulo P, Therneau TM, Jorgensen A, DeSotel CK, Egan KS, Dickson ER, Hay JE, Lindor KD. Bone disease in patients with primary sclerosing cholangitis: prevalence, severity and prediction of progression. J Hepatol. 1998;29:729-735. |
| 120. | Campbell MS, Lichtenstein GR, Rhim AD, Pazianas M, Faust T. Severity of liver disease does not predict osteopenia or low bone mineral density in primary sclerosing cholangitis. Liver Int. 2005;25:311-316. |
| 121. | Porayko MK, Wiesner RH, Hay JE, Krom RA, Dickson ER, Beaver S, Schwerman L. Bone disease in liver transplant recipients: incidence, timing, and risk factors. Transplant Proc. 1991;23:1462-1465. |
| 122. | Brandt DJ, MacCarty RL, Charboneau JW, LaRusso NF, Wiesner RH, Ludwig J. Gallbladder disease in patients with primary sclerosing cholangitis. AJR Am J Roentgenol. 1988;150:571-574. |
| 123. | Leung UC, Wong PY, Roberts RH, Koea JB. Gall bladder polyps in sclerosing cholangitis: does the 1-cm rule apply? ANZ J Surg. 2007;77:355-357. |
| 124. | Stiehl A, Rudolph G, Kloters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151-156. |
| 125. | Stiehl A, Rost D. Endoscopic treatment of dominant stenoses in patients with primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2005;28:159-165. |
| 126. | Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Waring JP, Fanelli RD, Wheeler-Harbough J. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58:475-482. |
| 127. | Bjornsson ES, Kilander AF, Olsson RG. Bile duct bacterial isolates in primary sclerosing cholangitis and certain other forms of cholestasis--a study of bile cultures from ERCP. Hepatogastroenterology. 2000;47:1504-1508. |
| 128. | Lee YM, Kaplan MM. Management of primary sclerosing cholangitis. Am J Gastroenterol. 2002;97:528-534. |
| 129. | Pohl J, Ring A, Stremmel W, Stiehl A. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2006;18:69-74. |
| 130. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. |
| 131. | Harnois DM, Gores GJ, Ludwig J, Steers JL, LaRusso NF, Wiesner RH. Are patients with cirrhotic stage primary sclerosing cholangitis at risk for the development of hepatocellular cancer? J Hepatol. 1997;27:512-516. |
| 132. | Wee A, Ludwig J, Coffey RJ Jr, LaRusso NF, Wiesner RH. Hepatobiliary carcinoma associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hum Pathol. 1985;16:719-726. |
| 133. | Kaya M, de Groen PC, Angulo P, Nagorney DM, Gunderson LL, Gores GJ, Haddock MG, Lindor KD. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol. 2001;96:1164-1169. |
| 134. | Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broome U, Chapman R, Fausa O, Egeland T, Rocca G. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205-1211. |
| 135. | Ahrendt SA, Pitt HA, Nakeeb A, Klein AS, Lillemoe KD, Kalloo AN, Cameron JL. Diagnosis and management of cholangiocarcinoma in primary sclerosing cholangitis. J Gastrointest Surg. 1999;3:357-367; discussion 367-368. |
| 136. | Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzen H. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327. |
| 137. | Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangio-carcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108:865-869. |
| 138. | Hultcrantz R, Olsson R, Danielsson A, Jarnerot G, Loof L, Ryden BO, Wahren B, Broome U. A 3-year prospective study on serum tumor markers used for detecting cholangio-carcinoma in patients with primary sclerosing cholangitis. J Hepatol. 1999;30:669-673. |
| 139. | Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangio-carcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734-1740. |
| 140. | Fevery J, Verslype C, Lai G, Aerts R, Van Steenbergen W. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci. 2007;52:3123-3135. |
| 141. | Brandsaeter B, Isoniemi H, Broome U, Olausson M, Backman L, Hansen B, Schrumpf E, Oksanen A, Ericzon BG, Hockerstedt K. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J Hepatol. 2004;40:815-822. |
| 142. | Ghali P, Marotta PJ, Yoshida EM, Bain VG, Marleau D, Peltekian K, Metrakos P, Deschenes M. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl. 2005;11:1412-1416. |
| 143. | Robles R, Figueras J, Turrion VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, Lopez P. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265-271. |
| 144. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. |
| 145. | Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458; discussion 458-461. |
| 146. | Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774-779. |
| 147. | Heimbach JK, Gores GJ, Nagorney DM, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma after aggressive neoadjuvant therapy: a new paradigm for liver and biliary malignancies? Surgery. 2006;140:331-334. |
| 148. | Rudolph G, Kloeters-Plachky P, Rost D, Stiehl A. The incidence of cholangiocarcinoma in primary sclerosing cholangitis after long-time treatment with ursodeoxycholic acid. Eur J Gastroenterol Hepatol. 2007;19:487-491. |
| 149. | Broome U, Chapman RW. Ulcerative colitis: sclerosing cholangitis today, cancer tomorrow? Gut. 1997;41:571-572. |
| 150. | Jayaram H, Satsangi J, Chapman RW. Increased colorectal neoplasia in chronic ulcerative colitis complicated by primary sclerosing cholangitis: fact or fiction? Gut. 2001;48:430-434. |
| 151. | Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. |
| 152. | Broome U, Lindberg G, Lofberg R. Primary sclerosing cholangitis in ulcerative colitis--a risk factor for the develo-pment of dysplasia and DNA aneuploidy? Gastroenterology. 1992;102:1877-8180. |
| 153. | Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522-525. |
| 154. | Loftus EV Jr, Sandborn WJ, Tremaine WJ, Mahoney DW, Zinsmeister AR, Offord KP, Melton LJ 3rd. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis. Gastroenterology. 1996;110:432-440. |
| 155. | Nuako KW, Ahlquist DA, Sandborn WJ, Mahoney DW, Siems DM, Zinsmeister AR. Primary sclerosing cholangitis and colorectal carcinoma in patients with chronic ulcerative colitis: a case-control study. Cancer. 1998;82:822-826. |
| 156. | Loftus EV Jr, Aguilar HI, Sandborn WJ, Tremaine WJ, Krom RA, Zinsmeister AR, Graziadei IW, Wiesner RH. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis following orthotopic liver transplantation. Hepatology. 1998;27:685-690. |
| 157. | Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, Brentnall TA. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89-95. |
| 158. | Pardi DS, Loftus EV Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. |
| 159. | Mir-Madjlessi SH, Farmer RG, Sivak MV Jr. Bile duct carcinoma in patients with ulcerative colitis. Relationship to sclerosing cholangitis: report of six cases and review of the literature. Dig Dis Sci. 1987;32:145-154. |
| 160. | Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol. 2007;31:907-913. |
| 161. | Bergquist A, Glaumann H, Persson B, Broome U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311-316. |
| 162. | Gossard AA, Lindor KD. Pregnancy in a patient with primary sclerosing cholangitis. J Clin Gastroenterol. 2002;35:353-355. |
| 163. | Christensen KL, Andersen BN, Vilstrup H. Primary sclerosing cholangitis with itching treated during pregnancy with ursodeoxycholic acid. Ugeskr Laeger. 1997;159:7151-7153. |