EARLY DAYS: 1940s-1950s
The recognition in the 1950s of the disease now known as autoimmune hepatitis (AIH) with its various guises and appellations is a richly interesting story, as hitherto recounted[12]. The history was recently embellished by Reuben in one of his memorable “Landmarks” published in Hepatology[3] (Figure 1). AIH, known in earlier days as chronic active hepatitis (CAH), was generally regarded as a “new” disease when reported in 1950 by Waldenstrom[4] in an out-of-the-way Conference Proceedings on nutrition. However, since it is hardly believable that any disease of autoimmune nature would have arisen then de novo, it is likely that it did exist but was unrecognized, for two reasons: first, autoimmunity in the 1940s had scarcely entered the medical mind and, second, most of the necessary diagnostic laboratory procedures (liver biopsy, serum aminotransferases, serum autoantibodies) were not then routinely available. The exception was the hallmark feature of hyperglobulinaemia, recognized by Waldenstrom[4], and also by others writing at around the same time (see below). Indeed increased levels of serum gamma globulin, first ascertained by moving boundary electrophoresis, are well illustrated in cases of cirrhosis of the liver reported in the early 1940s[5]. In fact, early descriptions that would fit AIH are discernible under the non-committal names of subacute hepatitis or subacute hepatic necrosis: such cases were ascribed to non-healing infectious hepatitis. As one example, Himsworth[6] in his 1947 monograph alluded to:
Figure 1 Montage and legend as prepared by Dr.
Adrian Reuben for “Landmarks in Hepatology”[3]. A: Jan Gösta Waldenström (right) with Dr Göran Bauer, President of the Swedish College of Physicians, Stockholm, c.1989 (courtesy of Dr. Frank Wollheim); B: Henry George Kunkel, Paris, 1979, being informed of his naming for the Lita Annenberg Hazxen Award (courtesy of Dr. Eng M Tan); C: Ian Reay Mackay, on the occasion of his Retirement Symposium in 1987 organized by the Walter and Eliza Hall Institute (courtesy of the subject). Reproduced by courtesy of Dr. Reuben and Wiley and Sons Inc., publishers of Hepatology).
“the form of subacute hepatitis which appears to arise as such, without the patient ever having had acute illness suggestive of liver disease… jaundice being absent or so faint as to unnoticed… the conditions affects women more often than men… the patient may date her present illness from an acute infection, such as cystitis or bronchitis, some 1 or 2 years previously… since then she has never felt really well … rheumatic pains, without evidence of articular damage are often noticed… or she may delay attending until the condition has passed into the next stage of post necrotic scarring… a particular problem is why do nearly all cases of sub-acute massive necrosis inevitably progress…?”
Similarly, Zimmerman et al[7] in 1951 described a case of subacute hepatic necrosis in a 36-year-old man with slowly developing jaundice and extreme hyperglobulinaemia (87 g/L) and in fact reached for an immunological explanation, in commenting that:
“the initial injury causes an alteration in liver protein, which stimulates the formation of anti-liver antibodies. These newly formed antibodies produce more liver injury, thus releasing more altered liver protein, which again contributes to the vicious circle of continuing necrosis.”
The general interpretation in the 1940s of subacute or chronic hepatitis was that it was a sequel to, and a consequence of, an unresolved acute infectious hepatitis. For example Neefe[8], writing from a long experience of viral hepatitis, comments in his review in 1946 on the occurrence of chronic hepatitis as a sequel of viral hepatitis. The stage was actually set for the later nomenclature of CAH in 1945 by Barker, Capps and Allen[9] who followed the course of viral hepatitis in military personnel in the Mediterranean theatre. They defined “non-recovery” as persistence of symptoms after 4 mo and applied the term chronic hepatitis “without any implications regarding the nature of the pathologic process or the eventual prognosis”. Further, their cases of “chronic hepatitis” were divided into active if symptoms were present, or inactive if there were only laboratory defined abnormalities[10]. Thereafter CAH became the standard descriptor of later years, albeit for a disease quite different from that exhibited by the soldiers. Even so, there still remained the strong impression that the precursor of CAH was hepatitis virus infection, according to articles by Wood and colleagues[11] in Melbourne in 1948, Kunkel and Labby[12] in New York in 1950, and Liebowitz[13] in New York in 1950 who followed up 68 patients with presumed acute viral hepatitis and reported that seven (11%) developed CAH. Waldenstrom[4] in his 1950 report had been more circumspect in specifying that the cause of the disease was unknown, wie wir alle wissen, although he also speculated on persisting viral infection (see below).
In the event, the disease seen during 1948-1950 that fulfils the modern description of AIH is illustrated by key phrases reproduced from the 1950 report of Waldenstrom[4], and by a 1951 Meeting Abstract of Kunkel and colleagues[14]. According to Waldenstrom:
“Dazu kommt bei fast allen Fällen das Auftreten von sog. Sternchen (Naevi aranei) und bei den Mädchen auch eine besondere Tendenz zur schweren Akneeruption. Eine langdauernde Amenorrhöe ist charakeristisch, die wahrscheinlich anovulatorisch ist… Es ist damit möglich, dass in der Zukunft eine Anzahl von diesen Fällen mit ACTH verbessert werden können… Die zweite Frage, ob die nachgewiesene Erhöhung des Gammaglobulins als Zeichen eines chronischen Immunisierungsprozesses aufzufassen ist, verdient meines Erachtens grösste Aufmerksamkeit… Die Atiologie dieser chronischen Leber-leiden ist-wie wir alle wissen-immer noch unbekannt. Es werden toxische, infektiöse und Nahrungsfaktoren als Ursache angenommen… Es scheint sehr wohl möglich, dass die Gammaglobulinvermehrung als Symptom einer Immunisierung gegen das im Körper verbleibende Virus aufzufassen ist”. Kunkel and colleagues[14] used the following phrases:
“Total proteins ranged from 9-13 per cent…this rise was due entirely to gamma globulin increase… eleven of these twelve patients were females… the maximum age was 32…onset of disease was insidious… course was prolonged and either stationary or downhill… frequently marked by periods of high fever, arthralgia, and arthritis… remarkable degree of plasma cell infiltration in the liver… which diminished during the course of disease… the etiology of the syndrome remains unknown.”
This apparently new liver disease came under particular scrutiny in Melbourne following the report of Wood et al[11] referred to above, and particularly from the standpoint of clinical and histological correlations, described by Saint et al[15] in 1953, who noted:
“fairly well defined clinical features, a highly characteristic pattern of biochemical tests including hypergammaglobulinaemia, and a histological picture which seems to indicate active chronic inflammatory changes… in many cases an initial history of contact or even a history of typical acute infectious hepatitis was lacking… but little doubt existed that the liver disease was due to infection with a virus”.
This latter article followed the earlier nomenclature of Capps[10] in 1948 of active and inactive chronic hepatitis but used histological rather than clinical criteria to draw the distinction. Accordingly Saint et al introduced the term active chronic hepatitis[15] that for a while coexisted with CAH, although the latter eventually prevailed. The dire outcome was recorded as follows:
“the course was progressively downhill… jaundice became a permanent feature… bleeding episodes became more frequent… alternatively these patients became permanently bedridden owing to their dropsical enfeebled state, and finally lapsed into coma… the time or presentation until death has varied between six months and two years”.
Among the cases assembled by Saint et al[15], one stands out. This was a 36-year-old woman, described further in 1955 in a case study by Joske and King[16] as active chronic viral hepatitis with positivity for the lupus erythematosus (L.E.) cell test. Their report includes the following:
“liver biopsy showed the typical picture of an active chronic viral hepatitis… cellular infiltration with lymphocytes and plasma cells and fibroblastic activity…fairly numerous L.E. cells present… cortisone produced a dramatic improvement in her arthralgia… we may recall that Leonie (1954) found L. E. cells in ascitic fluid from a patient with hepatic cirrhosis… we suggest that the L.E. cell and related phenomena might be based either: (1) on an abnormality of the antibody-producing mechanism… or (2) on changes in red or white cells or their constituents… which modify their characteristic “self markers”.
The latter 3-4 lines can be reasonably attributed to the pen of FM Burnet who, at this time in 1955 had already turned his mind to immunological aberrations in disease states. Also noteworthy in the report are comments on the plasma cellular content of the inflammatory infiltration into the liver, and the improvement conferred by treatment with cortisone.
The L.E. (lupus erythematosus) cell effect requires a few lines here. L.E. cells had been discovered incidentally in the 1940s by Hargraves[17] in bone marrow preparations from patients with “collagen diseases” of the lupus erythematosus type, and were modestly reported after a delay of some years in 1948 in the Mayo Clinic Proceedings. Hargraves himself was quite surprised by the interest that his report aroused[18]. This interest intensified further with the discovery that the L.E. cell effect depended on a serum factor, of gamma globulin nature[19], which only a few years thereafter became recognized by various groups as an antinuclear autoantibody. An item of interest, unreported and transmitted as a personal comment from Hargraves to this author, was that among the initial patients in whom L.E. cells were detected was a young girl (PC) suffering from chronic hepatitis! The L.E. cell test soon became a surrogate marker for an autoimmune basis for a given disease and, as such, was deployed in Melbourne in the early 1950s as the single available routine laboratory indicator for multisystem autoimmunity-hence its application to cases of CAH.
LUPOID TO AIH
After my return to Melbourne from abroad in 1955, it took only a little time to identify several instances of CAH in young women with hypergammaglobulinaemia and a lymphoplasmacytic infiltration in the liver and, in each, a positive test for the presence of L.E. cells in blood. Then the case for an immunological derangement as the cause of the disease became even stronger because at the time, DC Gajdusek who was a visiting scientist to the Hall Institute was attempting to develop a diagnostic serological test for viral hepatitis based on a complement fixation (CF) reaction, using as antigen liver tissue obtained at autopsy from a patient with fatal acute viral hepatitis. However sera from cases of acute hepatitis were at most only weakly positive using this CF reaction, whereas sera from cases of CAH (inserted as disease controls) tested positive, not only using as antigen the virally infected liver tissue but also using normal liver as well[20]. We can recall that during the development of the Wassermann serological test for syphilis infection, Spirochaeta-infected tissue was used initially, but normal tissue was found to serve equally well to elicit a positive reaction.
The several patients with CAH and a positive L.E. cell test appeared to represent a unique disease entity since most had, additionally, extrahepatic disease expressions including arthralgia, rash, haemolytic anaemia and others, typically seen in cases of systemic lupus erythematosus (SLE) wherein sera also gave a positive autoimmune CF test. In a report to Lancet in 1957[21], the concept was developed thus:
“linking certain types of active chronic hepatitis and lupus erythematosus… possibly through the common factor of disturbed immunological response… this group of cases has been provisionally designated as ‘lupoid hepatitis’ since ‘lupus’ has now acquired a far broader significance than the original term suggests… we consider that immunological destruction of the host’s liver cells best explains the perpetuation of the hepatitis and progression to cirrhosis. If this is so, it would be rational to use therapeutic measures (e.g. cortisone therapy) designed to modify this process, and our experience suggests that cortisone is of benefit in this autoimmune hepatitis.”
The global response to this report was remarkable. There were journal reports of patients with lupoid hepatitis from far and wide and, as well, detailed case studies, by Bearn et al[22], Reynolds et al[23] and others[12]. Thereafter there arose controversy, still not completely resolved[324], as to whether “lupoid hepatitis” was intended to specify a particular form of CAH and thus a disease in the realm of hepatology, or a component of a multisystem syndrome in the realm of rheumatology. Admittedly, this dilemma was not helped by our earlier writings which attempted to distinguish cases of “lupoid hepatitis” from those of “CAH” in which L.E. cell positivity was not demonstrable[25]. The development of the immunofluorescence test for antinuclear antibody (ANA), and its application to patients with CAH[26] soon revealed that cases of “lupoid” and “ordinary” CAH were indistinguishable on important criteria such as biochemical indices of liver dysfunction, histological abnormalities in the liver, and responsiveness to immunosuppressive therapy. Also, by the early 1960s, it was clearly evident that the disease in all its guises was best accounted for by an autoimmune reaction in the liver and thus, in 1965, we suggested that the disease be named “autoimmune hepatitis[27], although this appellation did not become formally endorsed until 1993[28]. Meanwhile, lupoid hepatitis lived on for quite some years, having heuristic appeal in some jurisdictions and causing semantic grievance in others, to the extent that one publication was directed to establishing that lupoid hepatitis was a “non-entity” within the disease group known as CAH[29]. Actually our Clinic had already discarded a possible association between lupoid hepatitis and SLE by showing in 1959 that liver lesions in classic instances of SLE were relatively trivial and nondescript[30], and a serological distinction was forthcoming a few years later (see below).
THERAPIES FOR CHRONIC ACTIVE/AIH
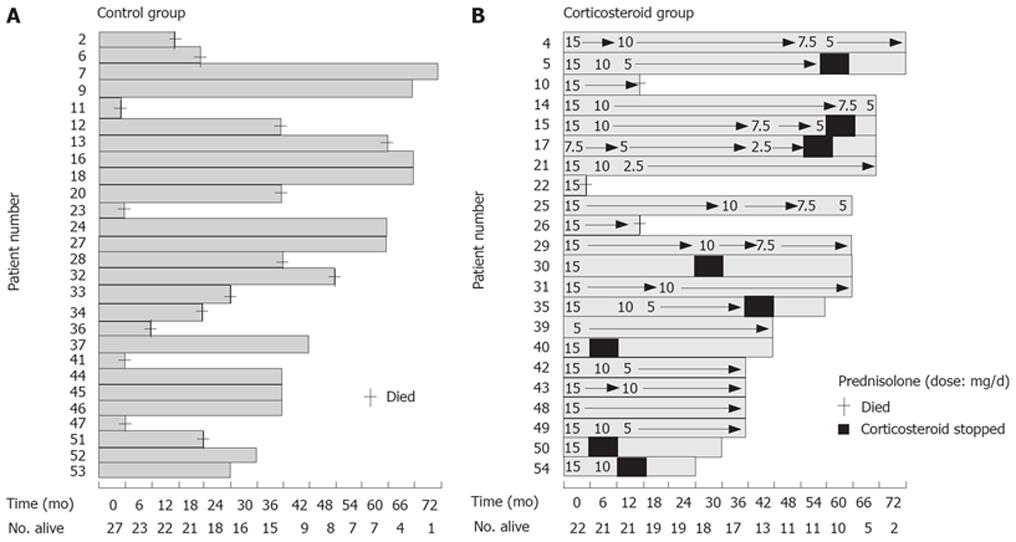
In our 1957 publication on lupoid hepatitis[21], the notion of immunological destruction of host liver cells was seen to provide a rationale for the use of “anti-immune” therapies to modify the process. Corticosteroid therapy in fact had been used in cases of CAH in Melbourne from as early as 1953, and, although initial results were equivocal[15], later experience proved more encouraging[31]. Also, Bearn et al[21] in their 1956 article that formalised observations made in the 1951 Meeting Abstract[14], reported that cortisone induced improvement in symptoms, and in physical and biochemical expressions of disease, and that withdrawal of cortisone from two patients resulted in prompt relapse. However they did retain the proviso that “there was no conclusive evidence that cortisone modified the disease process or that it will alter the eventual outcome”. A telling observation in Melbourne, reported in 1958[32], was that by serial daily monitoring of levels of serum aspartate transaminase after treatment was started, prompt falls in highly raised levels occurred, often within hours, and other indices of impaired liver function improved in turn. Moreover, since relapse tended to occur when cortisone was withdrawn, the need for long term maintenance therapy became evident. Hence co-therapy with a “steroid-sparing” agent was sought and, on theoretical grounds, there was chosen azathioprine, a derivative of the immunosuppressive drug 6-mercaptopurine, and this conferred added benefit[33]. However there was scepticism whether our routine prednisolone-azathioprine regimen, albeit symptomatically beneficial, actually altered the natural history of the disease. The view in Melbourne was that a trial that included randomly allocated placebo control patients was ethically problematic. Our decision was that all trial patients would receive active therapy after a 4-8 wk non-treatment run-up with close monitoring of multiple liver functional indices, and comparison would be made of mean levels for such indices for a group of 15 patients pre- and post -therapy with prednisolone, or prednisolone plus azathioprine. The outcome, published in 1968[34], led to the recommendation for long term (2-3 years) maintenance therapy with either prednisolone monotherapy, or combined prednisolone-azathioprine. However, conventional trials with randomly allocated non-treated controls were still called for, and these convincingly indicated survival benefits for the treated patients. The study of Cook et al[35] is exemplary, albeit with a substantial burden of mortality among the control group (Figure 2). Detailed studies on immunosuppressive therapy by the Mayo Clinic through the 1970s-1980s, with substantial case numbers, have shown sustained efficacy according to various criteria, survival, biochemical and histological[36].
Figure 2 Combined Figures 1 and 2 from a publication[35] on long-term prospective controlled trial of corticosteroid treatment of patients with active chronic (autoimmune) hepatitis.
The graphs show duration of treatment and survival for (A), left, non-treated control group and (B), right, corticosteroid treated group, and length of time (months) that the individual patients in either group had been in the investigation at the time of assessment. Numbers indicate the sequence of entry to the trial; +, time of death. Survival was 19/22 for the treated group and 12/27 for the control group. Reproduced with permission of Oxford University Press, publishers of Quarterly Journal of Medicine.
PATHOLOGICAL FEATURES OF CHRONIC ACTIVE/AIH
The increasing use of percutaneous biopsy of the liver in the 1950s greatly contributed to better knowledge of the nature of chronic hepatitis, well illustrated by the description in 1958 by Klatskin[37] of nine cases bearing the pre-1950s diagnoses of subacute hepatic necrosis/post-necrotic cirrhosis, and attributed to “anicteric infections with the hepatitis virus” because histopathological appearances met existing criteria for subacute hepatic necrosis of viral origin. However scrutiny of the clinical, biochemical and histological features strongly suggests that the illness in some, perhaps most, of the nine cases was actually AIH: there was female preponderance, hyperglobulinaemia, and corticosteroid responsiveness. However the particular feature of Klatskin’s study was the introduction of, or emphasis on, detailed histological features characteristic of those associated with autoimmune (as well as with viral) hepatitis. These included extensive “bridging” hepatocellular necrosis, ballooning of hepatocytes, regenerating hepatocytes with arrangement of cells in the form of “rosettes”, intense mononuclear inflammatory reaction; regenerative nodules, and acidophilic bodies (Councilman bodies) which are rounded intensely eosinophilic homogeneous cytoplasmic masses derived from hepatic cells undergoing coagulation necrosis. These latter appearances of course represent apoptosis of hepatocytes, recognized by Kerr[38] some 30-40 years before but not widely appreciated until interest developed in the 1980s in the apoptosis process. A detailed analysis of apoptosis in the context of chronic hepatitis and liver cell degeneration is given by Searle et al[39].
There were two morphological features additional to those described by Klatskin that deserve comment. One was the concentration of inflammatory activity and necrosis in the junctional region between the portal tract and liver lobule, initially described by Popper et al[40] as piecemeal necrosis, and currently referred to as interface hepatitis. The other feature was the striking accumulation among the inflammatory infiltrates of plasma cells, noted in most pathological descriptions, although not all[41], and earlier on leading to the disease being designated as “plasma cell hepatitis”[42]. This feature of CAH, i.e. prominence of plasma cells in liver, and bone marrow as well, aligns with the characteristic hypergammaglobulinaemia, although the antigenic specificity of these plasma cells and their secreted immunoglobulins has not yet been ascertained.
Finally we can note the protracted debate on whether histological features, irrespective of clinical diagnosis, would distinguish cases of chronic hepatitis with a progressive course and cirrhogenic potential-CAH-from those without such potential-chronic persistent hepatitis[4344]. After much elaboration in the 1970s these terms slowly disappeared from the lexicon.
HETEROGENEITY OF CAH
The term CAH used in 1950s and 1960s was generic, for what tended to be regarded as a single disease entity, with the prototypic cases being those that would fulfil criteria that presently define the autoimmune type of disease. However, impressions developed from the 1960s that CAH was not a homogeneous disease entity. Thus a collaborative study with colleagues in Singapore ascertained that CAH seen among Caucasians in Melbourne and Chinese in Singapore differed substantially, clinically, histologically and serologically[45]. Similarly, in unpublished observations in Melbourne, features of cases of chronic hepatitis and cirrhosis among recent Southern European immigrants differed from those among Australian-born individuals, such that we spoke of “Mediterranean cirrhosis”. An explanation was soon forthcoming. This depended on the fortuitous discovery by Blumberg and colleagues of an antigenic particle in serum initially called “Australia antigen” (Au), because it was first detected in serum from an Australian aboriginal serum donor[46]. Au was later found to be a surface protein of a virus identified with as the transmissible agent responsible for infectious (serum) hepatitis type B. The particle became known as hepatitis B surface antigen-HBsAg[47].
It became evident, as reported from several centres that cases of “CAH” segregated into those that were autoantibody positive and HBsAg negative, or vice versa and, in fact, prototypic (autoimmune) CAH became referred to as HBsAg-negative CAH[36]. Moreover, certain histological features of CAH, chiefly interface hepatitis, could be recognized in various other causally different cases chronic liver disease, whether due to viral infection, ethanol abuse, Wilson’s disease or other causes. An invitation was extended to me in 1972 to write an editorial on the “Prognosis (I changed this to Prognoses) of CAH” which led to a proposal that CAH was indeed heterogeneous in terms of aetiology, histological appearances, immunogenetic background, therapy requirements and outcome[48]. Moreover it seemed reasonable to assume that aetiological proportions among all cases of CAH would differ according to geographic region, and this proved to be the case, as judged by differences ascertained for cases of CAH in Australia and Singapore (see above) or Yugoslavia wherein proportions of cases due to chronic hepatitis B were substantially higher[49]. Finally, there were cases regarded as cryptogenic CAH, with some perhaps resulting from infection with non-A, non-B hepatitis virus; these later became attributable to the subsequently discovered hepatitis C virus (HCV). Currently, world-wide, the proportion of cases of chronic hepatitis B, followed by that of hepatitis C, far outnumber that of all other types.
All this led to the perception of a need for a clearer definition of CAH. This was met by the convening of a widely representative expert panel of hepatologists, the International AIH Group whose consensus deliberations led to reports in 1993 and 1999 on generally acceptable diagnostic criteria[2850].
GENETIC FEATURES OF CHRONIC ACTIVE/AIH
The wide regional differences in prevalence of different types of chronic hepatitis depend on differing environmental exposures and genetic composition of the particular population groups. Among the former, the endemicity of hepatitis virus infections is one important element.
The first genetic factor of interest in AIH was female predisposition, common to most autoimmune diseases, and for which the basis is still not well understood. Then, recognized in 1972, were genes that encoded for human leucocyte antigens, HLA. Cases of “classical” CAH, selected for typing for the then testable HLA alleles encoded at the HLA A and B loci, showed a significantly increased frequency of HLA-B8[51], an allele already implicated among Caucasian subjects with certain other autoimmune diseases. This association was soon confirmed in other centres, and was followed by the finding of an increased frequency in CAH of the D-related (DR) locus allele, DR3; a family study showed the combined inheritance of HLA A1, B8 and DR3 en bloc (a haplotype) from one or other parent, or both[52]. Possession of HLA DR3, particularly in those homozygous for these alleles, was predictive of a severe course and lesser responsiveness to immunosuppressive therapy[53]. The culprit allele is now styled as HLA-DRB1*0301. Later an additional HLA type, DR4 (HLA-DRB1*0401), not evident in our earlier studies, was identified[54]. The 6-7 fold risk for disease conferred by HLA DR3/4 is substantial but not highly potent meaning that, like all other “complex” autoimmune diseases, there must exist multiple other polymorphisms in “tolerance/ autoimmunity” genes that contribute to susceptibility: these are mostly undiscerned pending application of population genetics by genome wide screening.
IMMUNOSEROLOGICAL AND T-CELL STUDIES IN CHRONIC ACTIVE/AIH
The reactivities that initially (in the 1950s) were indicative of autoimmunity in CAH, the L.E. cell test and the AICF reaction, were soon superseded by more discriminatory and simpler laboratory assays. These are described in detail in other articles in this issue and in contemporary reviews[55–57].
Nuclear antigen(s)
Detection of ANA by indirect immunofluorescence (IIF) was introduced in the early 1960s[26] and remains the standard diagnostic screening procedure[57]. Superficially at least, the nuclear reactant(s) is the same as that responsible for the ANA reactivity observed in SLE i.e. the nucleosome (chromatin), although anti-DNA is much less frequent[56]. The idea that patients with AIH and SLE share one or more of the gene loci that determine ANA reactivity may be revealed by future population genome studies.
Smooth muscle antigen(s)
In 1963 there was observed a novel reactivity with smooth muscle of rodent gastric mucosa[58]. Detection of this smooth muscle antibody (SMA) to high titre proved to have high specificity for the diagnosis of CAH and notably, in “conventional” cases of SLE in which inflammatory destruction of liver cells is not evident, the test proved negative[59]. Further observations showed that some SMA+ve sera reacted by IIF with the mesangium of renal glomeruli, indicative of a wider distribution of the antigenic reactant than merely gastric smooth muscle tissue[60]. A subsequent observation was that some positive sera gave reactivity only with blood vessel walls (SMAv), and others reacted as well with renal glomeruli and renal tubular cells (SMAgt)[61]. The recognition that SMAv pointed to non-specific reactivity, and SMAvgt to reactivity specifically associated with AIH has led laboratory serologists to retain the designations SMAv and SMAgt in their diagnostic reporting.
The first indication of the identity of a reactant for SMA+ve sera was that reactivity could absorbed from serum by exposure to the cytoskeletal protein F-actin[62]. Further studies using IIF on cultured tissue cells revealed that SMA+ve sera stained cytoskeletal microfilaments (actin “cables”), representing polymeric F-actin, whereas SMA+ve sera from cases other than CAH stained intermediate filaments representing vimentin, desmin or others[63]. After much developmental work, there are now commercially available ELISA formats based on highly purified F-actin that have good specificity and sensitivity for the diagnosis of AIH[56]. The need at present is for better knowledge on the basis of anti-F-actin reactivity, including the significance (if any) for the pathogenesis of AIH, the epitope specificity of the antibodies, the relationship of epitopes to binding sites for the numerous F-actin binding proteins in the cell, and functional effects of anti-F-actin on cell motility[64].
LKM-1 antigen
In 1973, yet another serum reactant in AIH was discovered by IIF, to an antigen that was enriched in cytoplasm of liver and kidney proximal tubular cells[65]. This so-called liver-kidney “microsomal” (LKM) antigen, later designated LKM-1 because other LKM antigens became demonstrable[56], is actually located in the endoplasmic reticulum of liver and kidney proximal tubular cells[66]. A notable feature of anti-LKM-1-positive-AIH, versus ANA/SMA positive AIH, is that the serologically defined reactivities appear mutually exclusive, providing grounds for distinguishing these serological variants of AIH as type 2 and type 1[67]; this distinction has been retained by hepatologists even though few other differences exist. The point of interest heuristically on the serological distinction is that the respective autoantibody responses (ANA/SMA, anti-LKM-1) cannot be ascribed simply to (hepato) cellular injury, an explanation often levelled for the appearance of at least for some types of autoantibody. Although cases of anti-LKM-1-positive AIH are numerically far less than the traditional type, the ratios being about 1:10 in adults and 1:4 in children, type 2 AIH has proven far more amenable to investigation, since the LKM-1 antigen has been molecularly identified by screening a gene expression library as the cytochrome P450 isoform 2D6, enabling epitopes to be mapped[68]; there is demonstrated a CD4+ T-cell responsiveness to peptide antigens of CYP450 2D6[69]; and an experimentally credible model in mice has been developed[70].
Soluble liver/pancreas antigen
A soluble cytoplasmic antigen was independently discovered by CF[71], and ELISA[72], using pancreas or liver cell extracts respectively, and the reactant was found to be identical; it is generally known as “soluble liver/pancreas antigen (SLA). Sero-positivity, initially thought to identify a type 3 AIH, occurs in cases of AIH that are negative for other reactivities, but also in sero-positive cases of type 1 AIH. SLA has been cloned, identified and purified[56], and commercially available ELISA kits are diagnostically reliable. The pathogenetic significance of SLA is uncertain.
A liver-specific and disease specific antigen
This has been long searched for, since AIH behaves very much like an organ-specific autoimmune disease. In earlier times much effort was put into the characterisation of a preparation called liver-specific lipoprotein (LSP)[73], and assessment of the autoantigenic potential of this claimed liver-specific molecule[74]. Stemming from this was the recognition of the liver-specific membrane antigen, the asialoglycoprotein receptor[75], but this has not quite fulfilled the earlier hopes[76]. The liver cell membrane has been repeatedly studied, mostly by immunoblotting using AIH sera, for a molecular signal corresponding to a specific autoantigenic moiety, with the consistent finding being that of multiple reactive components of mw ranging from 20 to > 100 kDa[7778], but with none of these reaching candidate status as a liver-specific or disease-specific autoantigen.
CONCLUSION
Despite weighty circumstantial evidence for autoimmunity as one proximal cause of chronic hepatitis, and the existence of diagnostic serological reactants of high sensitivity and specificity, there is a disconcerting lack of mechanistic immunological explanation for AIH, and particularly the more prevalent type 1. There are high expectations for “the way we hope to be”[79] but, for these to be realised, new paradigms will be needed to explain AIH as well as other examples of organ-specific autoimmunity with non-organ-specific immune-dependent accompaniments.