INTRODUCTION
Malnutrition in general ward patients is associated with a prolonged length of stay[1], and increased infective complications[2,3]. Evidence that nutritional support of critically ill patients results in improved outcome is, however, limited. Descriptive studies indicate that underfeeding in the critically ill may be associated with an inability to wean from mechanical ventilation[4] and an increase in complications, particularly infections[5]. It is not clear whether these effects are causal or relate to an inability to achieve nutritional goals in patients with increased illness severity. However, severe underfeeding (less than 25% of requirements), increases the risk of nosocomial blood stream infections independent of illness severity[6]. In addition, the implementation of an algorithm to improve nutritional delivery in intensive care showed that this approach not only improved the provision of nutrition, but was also associated with a decreased hospital length of stay and a trend to decreased hospital mortality[7]. Despite the lack of unequivocal benefit on mortality, nutritional support is an accepted standard of care.
All of the feeding approaches used in the critically ill are associated with potential complications. Enteral nutrition, usually via a nasogastric tube, may lead to gastro-oesophageal reflux, with both overt and micro pulmonary aspiration, which potentially increases the risk of nosocomial pneumonia[8,9]. Total parenteral nutrition (TPN) on the other hand is associated with complications due to the insertion and presence of a central line, sepsis, increased cost and possible bacterial translocation across an atrophic gut mucosa. While TPN readily provides full nutritional support, enteral nutrition is less successful and only 50% of patients’ nutritional goals are met using the enteral route of nutrient delivery[6,10-12]. Inability to achieve an enteral feeding target is commonly due to cessation of feeds, with the decision to stop mostly due to delayed gastric emptying. In clinical practice this diagnosis is based on large gastric residual volumes (GRV)[11], despite a paucity of evidence to support this decision making process. The accuracy of GRV estimation and its use in feeding protocols is contentious (see below). Nonetheless enteral nutrition is currently considered to be superior to TPN, and early successful enteral nutrition to be best clinical practice[13]. Improving the success of nasogastric administration of nutrient requires an understanding of the gastrointestinal disturbances that underlie slow gastric emptying and developing strategies to treat these.
Current knowledge of these dysfunctions is incomplete, but recent studies have gone some way towards clarifying the mechanisms responsible for impaired nutrient delivery.
UPPER GASTROINTESTINAL MOTILITY IN HEALTH
Appreciation of the pathophysiology observed in critical illness requires an understanding of normal gastric and small intestinal motility. In health, the rate of gastric emptying is regulated via an integration of motor activity[14,15]. These motor patterns are determined by a mixture of neural and humoral mechanisms which modulate intrinsic myogenic activity. Regular depolarisation, which varies in frequency according to the region of the gut, is initiated by “pacemaker” activity from a network of cells embedded in the GI tract called interstitial cells of Cahal. Whether or not electrical activity initiates mechanical contraction, and the amplitude of such contractions, is determined by the influence of neural and humoral factors[16]. Extrinsic neural influence is largely mediated by the vagus, with parasympathetic effects causing an increase in motility. Sympathetic innervation from the prevertebral ganglia is inhibitory. A large number of hormones including cholecystokinin (CCK), peptide YY (PYY), motilin, glucagon like peptide-1 (GLP-1) and ghrelin are involved in regulation of this gut motility.
The motor activity of the gut differs between the fasting and fed state. Fasting motility is divided into three phases which migrate along the upper gut (the migratory motor complex); phaseIis characterised by quiescence, phase II by irregular contractile activity and phase III by periods of regular contractions sometimes referred to as the activity front[17]. Propulsion of luminal contents occurs mainly during late phase II or phase III, minimising stagnation of contents and subsequent bacterial proliferation. The precise regulation of MMC activity is unclear, but phase III activity can be triggered by stimulation of motilin receptors.
Ingestion of nutrients results in a postprandial pattern of activity. The proximal stomach acts as a reservoir (and provides a pressure gradient to control delivery of nutrient to the distal stomach) with the fundus relaxing in response to small intestinal nutrient. Subsequently, the fundus undergoes slow sustained contractions, which may act to distribute contents distally and is believed to have a major role in the control of liquid emptying[18,19]. Post-prandial activity in the distal stomach is characterised by irregular contractions which aid mixing and propagation of nutrient along the gastrointestinal tract. Mixing is due to intermittent isolated antral waves contracting against a closed pylorus. Gastric emptying of nutrient occurs predominantly in a pulsatile fashion when peristaltic wave activity continues through an open pylorus, aiding movement of contents into the duodenum[19,20]. The peristaltic wave is dependent on the integration of motor activity in the proximal and distal stomach, as well as the proximal small intestine[14,21,22].
The distal stomach and proximal small intestine behave as a functional unit and regulate gastric emptying of solids and liquids. This antro-pyloro-duodenal region is characterised by patterns of motility. These patterns include intermittent isolated pressure waves which vary in frequency and amplitude and coordinated propagated (peristaltic) pressure waves which migrate for variable distances through the antrum, across the pylorus and into the duodenum[23]. Transpyloric flow occurs as a result of both peristaltic and non-peristaltic antro-duodenal gradients[20,23]. The rate of transpyloric flow is regulated by feedback from receptors in the small intestine[24], where nutrient triggers neurohumoral responses resulting in reduced antral waves and increased isolated pyloric motor activity. In addition to the variation in isolated waves there is a reduction in antegrade propagating pressure waves[25-27]. This results in decreased transpyloric movement of nutrient and limits its delivery into the small intestine to 2-3 kcal/min[14,28,29].
UPPER GASTROINTESTINAL MOTOR DYSFUNCTION IN CRITICAL ILLNESS
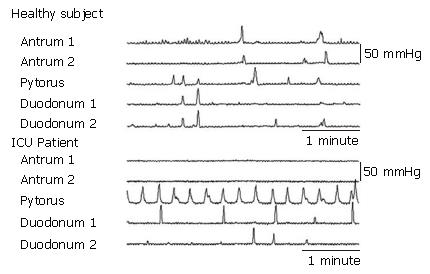
Delayed gastric emptying is common in the Intensive Care Unit, occurring in approximately 50% of mechanically ventilated critically ill patients[30-35]. In these patients both fasting and fed motility of the upper GI tract are frequently impaired[36,37]. There is a virtual absence of gastric phase III motility during the fasting state, although the frequency of phase III activity in the duodenum appears normal[38] perhaps reflecting a loss of integration within the antro-pyloro-duodenal unit. During feeding, however, a number of additional abnormalities become apparent. These include delayed fundal relaxation, prolonged recovery[39], reduced antral motility[36,38] and increased isolated pyloric activity (Figure 1). These occur when the small intestine is exposed to even low levels of nutrients and are likely to result in delayed gastric emptying[36]. Thus delayed gastric emptying may reflect hypersensitivity to small intestinal nutrient. In contrast to delayed fundal relaxation and impaired antral motility, duodenal activity usually persists[36,37] (although the organisation of duodenal activity is frequently abnormal)[40] and may explain why, in the absence of prokinetics, post pyloric feeding may potentially be more successful than gastric enteral nutrition.
Figure 1 Manometric tracing from healthy and critically ill subjects during duodenal feeding (1 kcal/min).
Location of pressure sensors is shown on left. In the healthy subjects antral and duodenal peristalsis is seen, whilst the increase in activity in the pylorus in the critically ill is associated with absent antral activity[36].
The mechanisms underlying motor dysfunction in critical illness are uncertain. However, concentrations of CCK and PYY, which normally increase when nutrient reaches the small intestine in health, are markedly increased in critical illness especially in those intolerant of enteral feeding[41,42]. It is, therefore, possible that CCK and PYY may mediate the enhanced entero-gastric reflex described above.
Inflammatory cells in the intestinal wall contribute to motility disturbances after surgery. Surgical manipulation of the small intestine activates macrophages to release cytokines and cause an additional leukocyte response in the muscularis externa[43]. In murine models this has been suggested to contribute to delayed gastric emptying[44]. However, while inflammation from a local insult has been shown to cause altered motility in the post-operative setting, the impact of inflammation from systemic non-operative insults is unknown. The effect of inflammation on motility in critical illness warrants further investigation.
RISK FACTORS FOR FEED INTOLERANCE
The aetiology of upper gastrointestinal motor dysfunction in critical illness is unclear, but is probably multifactorial. Potential factors implicated include the admission diagnosis, pre-existing illnesses, electrolyte abnormalities (such as hyperglycaemia), age, gender, drugs (such as narcotics or catecholamines), recent abdominal surgery, shock, and circulating cytokines[45]. Illness severity, quantified as an APACHE II score (calculated using age, physiological variables and chronic health conditions) also correlates with delayed gastric emptying[46].
Admission diagnosis
Gastric emptying data suggest that there are high risk groups for feed intolerance. These include patients with burns[32,33], head injuries[34], sepsis and multi-trauma[46]. However, patients with burns who are fed early and aggressively have a low incidence of feed intolerance[47] implying that earlier feeding is protective.
Premorbid conditions
Patients with premorbid disordered glucose metabolism are frequently admitted to the ICU[48] and delayed gastric emptying occurs commonly with diseases such as diabetes mellitus. However, critically ill patients with pre-existing type 2 diabetes appear to have normal or even rapid gastric emptying of liquid nutrient compared to non-diabetics[49]. Faster emptying in diabetics may initially appear counter intuitive, but in non-critically ill diabetic patients, gastric emptying of liquid nutrient is also quicker when compared to volunteers without diabetes[50-52]. This is in direct contrast with the delay in gastric emptying of solid or semi-solid meals, in diabetics. As previously mentioned the proximal stomach contributes to normal gastric emptying. Critically ill patients without diabetes have impairment of proximal gastric relaxation leading to delayed emptying. However, in the critically ill patient with pre morbid type 2 diabetes, the proximal stomach relaxes, distends and accommodates a larger volume during infusion of duodenal nutrition, which is a response that mirrors what occurs in normal healthy physiology[53,54]. In addition, critically ill diabetic patients have preservation of fundal waves while the non diabetic critically patients have decreased frequency of isolated waves in the fundus. Fundal waves may aid progression of nutrient in critical illness[53]. The exact mechanism causing this “pseudo normalization” of gastric motility and gastric emptying is unknown, but may be related to autonomic neuropathy in the diabetic patient, potentially causing a loss of the enhanced enteric feedback process that is common to critical illness.
Increasing age has been associated with a slowing of gastric emptying in healthy volunteers[55,56]. As ICU patients are, in general, older than the non-hospitalised population, it would be anticipated that age might contribute to the delay in gastric emptying seen in critical illness. Elderly critically ill patients are at increased risk of delayed emptying compared to younger patients[33,46]. Gender has also been reported to impact on gastric emptying in health, with women having slower emptying rates compared to men[57-59], although this association, unlike age, does not seem to apply in the critically ill[33].
Electrolyte abnormalities
Hyperglycaemia occurs frequently in critically ill patients, even in patients with normal baseline glucose homeostasis[48]. In health, hyperglycaemia impairs gastrointestinal motility and gastric emptying[60], so it follows that the critically ill also have an association between hyperglycaemia and delayed gastric emptying, with a subsequent effect on feed intolerance[61]. As discussed previously, eugylcaemic diabetic patients do not have an increased incidence of delayed gastric emptying and feed intolerance.
Drugs
Many drugs used in the critically ill can potentially influence gastrointestinal motility. Of particular concern are sedatives, analgesics and vasopressor agents. Both endogenous and administered opiates, acting via mu receptors, may disrupt upper gastrointestinal motility[62-66]. The impact of opiates, on the gastrointestinal tract, is due to both central effects and peripheral opioid receptors located in the gut. Low dose epidural morphine delays gastric emptying[67] and causes disordered motility[66] implying a central effect is important. The effect of receptor agonism is additive when both spinal and parenteral morphine are administered together[65]. Opiates slow gastric emptying as a result of decreased gastric tone[64] and antral contractions[68] with retrograde duodenal activity[66]. Although opiates have been associated with delayed gastric emptying in the critically ill[33], similar abnormalities have also been observed in critically ill patients not receiving exogenous narcotics[36]. Using propofol as a sedative in the critically ill is considered by clinicians to cause less slowing of gastrointestinal function. This belief is based on studies in healthy people; where low doses of propofol appear to have limited effects on gastric emptying in healthy humans[69,70] and because using propofol in combination with morphine attenuates the decrease in gastric tone usually seen when the opiate is used as a single agent. However, the evidence is far from conclusive. While propofol improved gastric tone during morphine administration, it had no effect on actual gastric emptying[64] and in animal models propofol prolonged phase 1 motility during fasting in pigs[71]. The effects of propofol on gastrointestinal motor function may be dose related as the drug, at anaesthetic doses, reduces gastric emptying and increases intestinal transit time in mice[72]. In humans it remains difficult to accurately compare sedative agents in critically ill patients, as intensivists have usually avoided a propofol based sedative regime in the more acutely ill population because of concerns with hypotension and the likelihood of prolonged sedative use. No prospective comparison of propofol and morphine, adjusted for illness severity, has confirmed superiority of propofol on gastric emptying. In addition, propofol has been associated with feed intolerance in head injured patients[73] and it may be prudent to avoid a dogmatic belief in the benefits of propofol for feeding tolerance. Midazolam, a benzodiazepine often prescribed with an opiate as a combination sedative in the ICU, also reduces gastric emptying and prolongs gastrointestinal transit[72].
High concentrations of circulating catecholamines, either endogenous or exogenous, are common in critically ill patients. Adrenaline reduces gastric emptying by a beta-adrenergic effect[65]. This is likely to be a class effect, as low dose dopamine compared to placebo adversely affects gastroduodenal motility in the critically ill[66]. In addition, high dose catecholamines have been associated with a reduction in the prokinetic effect of erythromycin[45].
IDENTIFICATION OF FEED INTOLERANCE & DELAYED GASTRIC EMPTYING
Gastric emptying is rarely directly measured in the critically ill other than for research purposes. Regular measurement of gastric residual volume (GRV) during the infusion of enteral nutrition has been considered a convenient clinical tool by many clinicians and is used as a surrogate to indicate gastric emptying, success of feeding and potential risk of aspiration. Despite acceptance of GRV in feeding protocols by the majority of ICUs, the utility and significance of this measurement is controversial as it is dependent on a number of factors. These include the position of the tube, tube characteristics (such as tube type and number of openings), the volume of syringe used[74] and the operator performing the test[75]. GRV is usually performed every 4-6 h and, unlike the continuous monitoring of other end organ function, the significance of a ‘snap shot’ or a one off value may be hard to interpret. In addition, the relationship between GRV and gastric emptying is weak[32,35,74]. These factors have lead to a lack of consensus on an acceptable value for GRV during enteral feeding. Computer simulated modelling suggests that GRV should plateau between 232 and 464 ml during enteral feeding at a rate of 25-125 mL/h[76]. Currently the majority of intensive care units have protocols for feeding that consider a change in delivery rate or site if the GRV is between 150-400 mL/s. The evidence for this is limited, as 25% of patients with a GRV > 150 mL have normal gastric emptying and can continue to be fed successfully without prokinetics[77]. In addition, and of more importance clinically, is that the high rate of aspiration and oesophageal regurgitation observed in these patients is independent of GRV[78]. As the current clinical significance of GRV is uncertain a convenient, continuous and more reliable test of gastric emptying would assist in the nutritional management of these patients.
TREATMENT OPTIONS
Failure of delivery of nasogastric nutrition is usually managed either by pharmacological intervention or a change in the route of delivery of feeding. Various prokinetic agents are available but few, to date, have been studied in depth in critically ill patients.
PHARMACOTHERAPY
Metoclopramide
Metoclopramide is a dopamine receptor antagonist with central and peripheral effects, as well as weak 5-HT3 receptor antagonism and 5-HT4 agonism[79]. The drug releases acetylcholine from gut neurones, antagonising the inhibitory effect of dopamine on gastrointestinal motility. Jooste et al[80] demonstrated that metoclopramide improved gastric emptying in critically ill patients. However, recent data[81,82] have shown that in critically ill patients rapid tachyphylaxis occurs, such that at 7 d, only 25% of patients given metoclopramide will continue to be fed successfully. The recommended dose of metoclopramide is 10 mg TDS or QID as there is limited evidence of improved efficacy at higher doses in the critically ill. Without supporting evidence it remains prudent to limit the dose administered in the non-research setting because of the neurological side effect profile of the drug. Metoclopramide is ineffective as a prokinetic in head injured patients[73] and potentially has a deleterious effect in patients at risk of raised intracranial pressure[72]. As such other agents are preferred in patients who have suffered a significant neurological insult.
Erythromycin
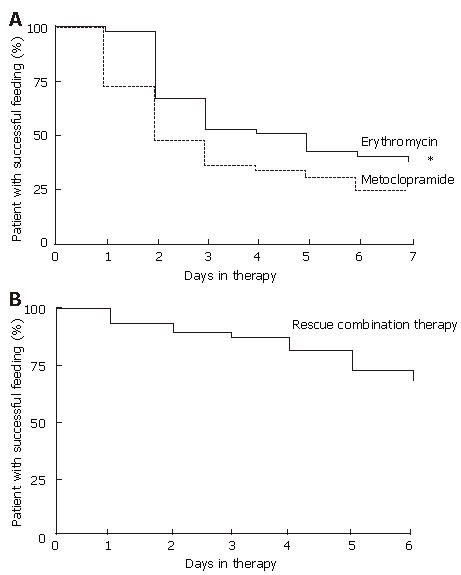
The macrolide antibiotic, erythromycin, when administered in sub-antibiotic doses (70-250 mg), acts as a motilin agonist and stimulates gastric motility. Motilin receptors are found in abundance in the gastric antrum and proximal duodenum and induce contractions in the gastrointestinal tract. Intravenous erythromycin increases antral motility and accelerates gastric emptying in unselected critically ill patients[83] and reduces GRV in critically ill patients with feed intolerance[84]. Erythromycin is a more effective prokinetic than metoclopramide in this patient group[81,82]. However, as with metoclopramide, its efficacy decreases over time so that after 7 d of treatment only about 45% of patients remain tolerant to nasogastric feeding. The combination of erythromycin and metoclopramide is superior to either drug alone with less tachyphylaxis (Figure 2). Using a combination of the two drugs, 70% of patients can be successfully fed by nasogastric tube at 6 d[82]. Enthusiasm for the use of erythromycin is tempered by fears of cardiac toxicity and bacterial resistance. Clinicians should remain vigilant to drug interactions in critically ill patients, as patients may be on multiple drugs (such as amiodarone and haloperidol) that predispose to a prolonged QT interval. It is likely that cardiac toxicity is minimised by using low dose therapy. A recent report by Ritz et al[85] demonstrated 70 mg erythromycin IV to be as effective as 200 mg in improving gastric emptying in the critically ill. Currently intravenous erythromycin is available in 500 mg ampoules and it may be easier to administer 100 mg rather than 70 mg at the bedside. The optimal timing between doses has not been clarified but is probably between BD and QID. Bacterial resistance remains a concern, regardless of dosing schedule, and investigation of motilin agonists without antibiotic effect or other unrelated agents is warranted.
Figure 2 Kaplan Meier plots comparing the effects of erythromycin (200 mg IV BD) and metoclopramide (10 mg IV QID) in the treatment of feed intolerance.
Significant tolerance developed to both drugs over 7 d. The combination of both drugs is effective rescue therapy[81].
Opiate receptor antagonists
As opiate administration may be an important cause of reduced gastrointestinal motility and unsuccessful feeding in the critically ill, opiate antagonists are logical options for treatment. To avoid antagonism of required analgesic and sedating properties naloxone has been administered via a nasogastric tube. Eight mg naloxone administered every 6 h, via nasogastric tube, reduced GRV in 84 mechanically ventilated patients who were receiving IV fentanyl. The treatment group also had a lower incidence of ventilator associated pneumonia (VAP)[86]. However, the decreased incidence of VAP in the naloxone group did not lead to a reduction in time to wean from mechanical ventilation, or time to discharge from ICU and the efficacy of naloxone requires confirmation. As naloxone is packaged in 400 μg ampoules, a dose of 8 mg involves the inconvenience of opening 20 ampoules 4 times a day. This restricts easy administration and increases expense, which may have limited the uptake of this approach. There has also been research into other mu receptor blockers, such as Alvimopan, a peripherial mu-opioid receptor antagonist. Importantly, unlike naloxone this agent does not antagonise opioid analgesia[87]. Alvimopan reverses the inhibitory effect of opiate on small bowel motility as measured by scintigraphy[88] and has been used successfully in postoperative patients to shorten both the time to bowel recovery and time to discharge from hospital[89]. However, it has not been formally assessed in critically ill patients.
CCK receptor antagonists
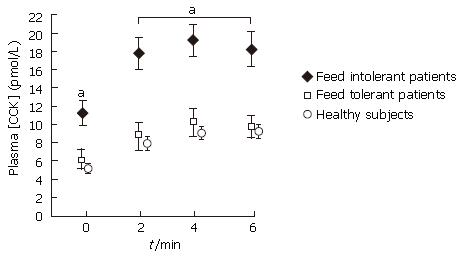
Elevated CCK levels inhibit gastric emptying and motility in health[90] and are associated with feed intolerance in critically ill patients (Figure 3)[41]. CCK1 receptor antagonists have been reported to improve lower oesophageal sphincter function and accelerate gastric emptying. This class of drug therefore has potential as an effective treatment for delayed gastric emptying and feed intolerance in the critically ill but this has not been examined.
Figure 3 Plasma CCK concentrations during fasting and duodenal nutrient stimulation in both feed tolerant and intolerant critically ill patients.
Plasma levels of CCK for feed tolerant patients match those seen in healthy subjects receiving intra duodenal nutrition. Feed intolerant patients have higher levels whilst fasting and during enteral nutrition compared to feed tolerant (aP < 0.01)[41].
5-HT4 receptor agonists
Serotonin (5-Hydroxytryptamine), a monoamine neu-rotransmitter, acts on a variety of receptor types in the gastrointestinal tract with the effect of serotonin depend-ing on the receptor type that is expressed[91]. As 5-HT4 stimulates peristalsis there had been interest in the use of these drugs as prokinetic agents, following their successful use in irritable bowel syndrome[92]. Tegaserod, a serotonin partial agonist, had been reported to increase gastric motility in critically ill patients[93]. However, in March 2007 the FDA requested Tegaserod be withdrawn due to an increase in cardiovascular side effects. The mechanism of this unclear, but reminds critical care clinicians to remain cautious when prescribing recently introduced drugs for off licence indications.
Ghrelin
Ghrelin is a natural ligand for the growth hormone (GH) receptor[94] and has strong GH releasing activity. A motilin related peptide, ghrelin has a number of other actions including stimulation of appetite (hence the label of the “fattening” peptide[95]), gastrokinetic effects and positive inotropic effect on the circulation[96]. The motility effects include induction of gastric phase III contractions as well as increasing the resting tone of the proximal stomach[94]. Ghrelin has been successfully used as a prokinetic in diabetic gastroparesis[97]. Treatment with an agent that has anabolic effects, improves gastrointestinal motility as well as providing circulatory support has inherent desirable properties. However, there are no data as yet on the use of ghrelin in the critically ill, and enthusiasm for exogenous administration of the peptide is tempered by previous studies where the use of GH in the critically ill was associated with increased mortality[98].
NON PHARMACOLOGICAL APPROACHES
Post pyloric feeding tubes
In patients with delayed gastric emptying successful feeding may be achieved with post pyloric feeding tubes[99]. Systematic reviews, however, have failed to demonstrate an impact of this practice on nutritional delivery or clinical outcomes after early initiation of post pyloric feeding[100,101]. Furthermore, in patients who fail nasogastric feeding, post pyloric tubes and prokinetic therapy are equally effective[102]. Thus current recommendations are that post pyloric feeding should be reserved for patients who fail nasogastric feeding and do not respond to prokinetics.
Enthusiasm for post pyloric nutrition is moderated by the lack of an accepted technique to place the feeding tubes. Ideally, the technique would be easy to learn, have a high success rate, incur minimal cost and require only portable equipment so that the patient need not be transported out of the unit. Passing feeding tubes into the duodenum is difficult in the critically ill and rapid placement usually requires fluoroscopic or endoscopic support. Access to either technique is limited in many Intensive Care Units. Insertion of post pyloric feeding tubes, without endoscopy or radiological imaging, has been reported using various proprietary tubes and techniques. In general such devices either rely on self-migration or magnetic guidance. The Tiger TubeTM (Cook Medical) has small plastic tabs which allow the tube to be captured by peristalsis and carried distally. Anecdotal reports suggest it is faster than blind placement by the bedside. A large multi-centre study is currently recruiting in Australia that will compare nutrient delivery with a post pyloric Tiger Tube against standard nasogastric feeding. However, concerns have been expressed about the potential for peri-procedure bleeding and there has been published reports of mucosal damage[103]. It would seem prudent to be cautious about long term placement, as well as insertion or removal of the tube if a coagulopathy is present. The GabrielTM feeding tube (Syncro Medical Innovations) uses a magnet to guide the tip of the tube through the pylorus. Another electromagnetic device is the CorTrakTM (Viasys Medisystems) which relies upon an electromagnetic field transmitted from the catheter tip measured by a receiver placed unit on the patient. This signal is transferred to a graphic display to guide and confirm placement. These two techniques appear safe, and are easy to use, with high success rates of tubes rapidly entering the duodenum[104-106]. An alternative approach uses erythromycin, air insufflation and stomach ECG[107]. This has achieved post pyloric placement success rates (up to 90%) that are equivalent to the proprietary tube approaches. This approach is more economical than proprietary tubes, endoscopy or fluoroscopy.
CONCLUSION
Provision of adequate nutrition in critical illness is generally accepted as desirable and early feeding is considered superior to delayed feeding. The enteral route is preferred as it is cheaper, and may be associated with less sepsis; however, the high incidence of gastric and small intestinal dysmotility slows gastric emptying and often limits nasogastric delivery of nutrients. Although in widespread use, gastric residual volumes are an unreliable measure of gastric emptying, may underestimate tolerance to nasogastric feeding and do not predict complications such as regurgitation and aspiration. Delayed gastric emptying probably results from disturbances in the frequency and organisation of contractions in both the proximal and distal stomach. Increased neuro-hormonal feedback in response to relatively small amounts of nutrient in the small intestine, possibly mediated by CCK may contribute to this motor dysfunction. Awareness of patients at risk for delayed gastric emptying and pre emptive management may decrease the incidence of feed intolerance amongst critically ill patients. The current best treatment of patients who fail nasogastric feeding is prokinetic therapy. A combination of erythromycin and metoclopramide may reduce the common problem of tachyphylaxis. The dose of erythromycin to promote motility may be smaller than previously appreciated and could alleviate some concerns relating to adverse side effects. If prokinetics fail, delivery of post pyloric nutrition should be considered. Changes in nutritional management in the future may include better means to identify patients at risk of delayed gastric emptying, accepting higher gastric residual volumes, and the use of nutrients designed to optimise gastric emptying. Novel agents, including antagonists to CCK or opiates, as well as agonists of grehlin or motilin, need further investigation.
S- Editor Ma N L- Editor Rippe RA E- Editor Liu Y