Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1362
Revised: July 1, 2005
Accepted: August 26, 2005
Published online: March 7, 2006
AIM: Prednisone and azathioprine represent the standard treatment for autoimmune hepatitis (AIH). However, only 65% of the patients enter complete histological remission. Recently, budesonide (BUD) was reported to be a promising alternative. In this study we assessed the efficacy and safety of BUD in AIH.
METHODS: Eighteen patients (12 women, 6 men; mean age 45.4 ± 21 years) with AIH were treated with BUD (Budenofalk®) 3 mg thrice daily and followed up for at least 24 wk. Seven patients also had features of primary biliary cirrhosis (n = 5) or primary sclerosing cholangitis (n = 2). Advanced liver fibrosis or cirrhosis was present in 6 patients.
RESULTS: Fifteen (83%) patients had a complete clinical and biochemical remission. Ten patients, including five with acute hepatitis, were given BUD as first-line therapy, of which seven enter remission. Three patients, two with liver cirrhosis, did not improve. All patients with second-line therapy experienced long-term remission. A histological remission was also seen in three patients. Clinically relevant BUD-induced side effects were recorded only in patients with liver cirrhosis (n = 4).
CONCLUSION: BUD is effective in remission induction in the majority of our patients with AIH. Side effects and treatment failure was mainly observed in patients with liver cirrhosis.
- Citation: Csepregi A, Röcken C, Treiber G, Malfertheiner P. Budesonide induces complete remission in autoimmune hepatitis. World J Gastroenterol 2006; 12(9): 1362-1366
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1362
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease characterized by a female preponderance, hypergammaglobulinemia and circulating non-tissue specific autoantibodies. AIH usually responds to immunosuppressive therapy; and prednisone (PRD) with or without azathioprine (AZA) is the treatment of choice. This results in histological remission in approximately 65% of the cases. Several patients become intolerant to this regimen and in some it is even contraindicated[1]. Thus, new first-line and salvage therapies are needed, and several drugs are currently under investigation[2].
Budesonide (BUD), a nonhalogenated glucocorticoid, is of particular interest for the treatment of autoimmune liver diseases: it has a 15-fold greater receptor binding capacity than PRD, and a high hepatic first-pass clearance, exceeding 90% of the orally administered dose. Hence it was reported to be free of systemic effect in patients without advanced liver fibrosis[3,4]. However, as yet, data of BUD for the treatment of AIH are very limited and controversial.
In our present study we evaluated the efficacy and safety of BUD as first- and second-line therapy for AIH.
Eighteen patients (12 women and 6 men; mean age 45.4 ± 21 years, range 21 and 68 years) were selected from a collective of fifty patients with the initial diagnosis of AIH and treated with BUD between January 2002 and July 2004. Selection was based on the following criteria:
Criteria for AIH were: (1) serum alanine aminotransferase (ALT) level at least three times of the upper limit of normal (ULN); (2) serum immunoglobulin G (IgG) level at least 1.5 times of ULN; (3) positive test result(s) for non-tissue or organ-specific autoantibodies (titer ≥ 80); (4) liver histology consistent with the diagnosis of AIH. Definite diagnosis of AIH required the presence of at least three of the four criteria or 16 points or more on aggregate score proposed by the International Autoimmune Hepatitis Group[5]. An "overlap" syndrome of primary biliary cirrhosis (PBC) and AIH was defined by the simultaneous association of these disorders.
Diagnostic criteria of PBC used in this study were: (1) a positive test for antimitochondrial antibodies (AMA) in serum (titer≥ 80); (2) serum alkaline phosphatase (ALP) level at least 2 times of ULN or gamma glutamyl transferase (GGT) activity at least 5 times of ULN; (3) a diagnostic or compatible liver histology. Two of these criteria were required for the diagnosis of PBC.
Eleven of our patients were categorized as AIH alone, and seven satisfied the revised international criteria for definite diagnosis of AIH (≥ 16 points) and five the probable diagnosis of AIH [5]. The median score of patients with AIH was 16 points (range, 11 to 22 points). Five patients presented also with features of PBC. Primary sclerosing cholangitis (PSC) was diagnosed in two patients who had elevated cholestatic and aminotransferase enzyme activities, concentric periductal sclerosis and interface hepatitis on liver biopsy, serum antinuclear antibodies and elevated IgG level (Table 1). The median score of patients with overlap syndrome was 13.6 points (range, 6 to 17 points).
| Patient (Gender/Age) | Diagnosis | IAHG Score | Autoantibodies (titer) | ALT (<0.85μmol/s.L) | ALP (<2.15μmol/s.L) | HAI | Fibrosis | Therapy | Res- | ||||||
| ANA | SMA | ANCA | AMA | (Ref. 6) | (wk) | ponse | |||||||||
| pre- | post- | pre- | post- | pre- | post- | ||||||||||
| Budesonide | Budesonide | Budesonide | |||||||||||||
| First-line therapy | |||||||||||||||
| B.M-L. (F/65) | AIH-PBC | 19 | 1280 | - | - | - | 15.18 | 0.22 | 7.12 | 1.44 | 5 | 3 | 3 | 241 | CR |
| D.B. (M/26) | AIH | 14 | 320 | - | - | - | 16.4 | 0.78 | 3.19 | 0.95 | 7 | nd | 4 | 36 | CR |
| D.C. (F/49) | AIH | 22 | 640 | - | - | - | 1.91 | 0.89 | 3.89 | 3.53 | 4 | nd | 5 | 24 | NR/AE |
| H-S.M. (F/31) | AIH | 17 | 160 | 80 | - | - | 4.43 | 0.6 | 1.62 | 0.9 | 7 | nd | 2 | 50 | CR |
| K.M. (F/62) | AIH | 14 | - | - | - | - | 3.45 | 3.59 | 7.86 | 6.29 | 5 | nd | 4 | 122 | NR |
| L.M. (M/60) | AIH | 16 | 640 | 640 | - | - | 4.1 | 0.35 | nd | nd | 1 | nd | 0 | 82 | CR |
| N.G. (F/64) | AIH-PBC | 12 | 2560 | - | - | 640 | 10.73 | 0.38 | 7.53 | 1.63 | 11 | 1 | 0 | 82 | CR |
| S.T. (M/40) | AIH | 12 | - | - | >80 | - | 8.39 | 0.57 | 7.84 | 1.36 | 1 | nd | 0 | 24 | CR |
| T.H. (F/50) | AIH | 19 | 640 | 160 | - | - | 3.38 | 6.77 | 1.54 | 1.74 | 9 | nd | 3 | 42 | NR |
| T.E. (F/49) | AIH-PBC | 15 | - | 640 | - | 1280 | 2.27 | 0.58 | 4.21 | 1.61 | 5 | 2 | 5 | 112 | CR |
| Second-line therapy | |||||||||||||||
| B.R. (M/39) | AIH | 14 | 160 | - | - | - | 4.42 | 0.6 | 2.16 | 0.63 | 6 | 2 | 1 | 100 | CR |
| B.S. (F/25) | AIH | 17 | 160 | - | - | - | 16.48 | 0.41 | 4.89 | 1.38 | 6 | 1 | 3 | 116 | CR |
| D.H. (F/61) | AIH-PBC | 14 | >2560 | - | - | -3 | 2.37 | 0.45 | 11.91 | 1.24 | 5 | 3 | 4 | 32 | CR |
| F.C. (F/41) | AIH | 17 | 2560 | 2560 | - | 320 | 10.1 | 0.55 | 4.6 | 0.88 | 6 | nd | 2 | 72 | CR |
| G.W. (M/68) | AIH-PBC | 6 | - | - | - | - | 28.65 | 0.18 | 4.1 | 0.77 | 3 | nd | 4 | 110 | CR |
| K.C. (F/40) | AIH | 16 | 640 | 160 | - | - | 10.1 | 0.68 | 4.6 | 1.77 | 4 | nd | 1 | 50 | CR |
| S.H-D. (M/64) | AIH-PSC | 11 | 2560 | - | - | - | 2.01 | 0.6 | 30.13 | 2.3 | 4 | 1 | 1 | 80 | CR |
| S.U. (F/41) | AIH-PSC | 14 | 1280 | - | - | - | 20 | 0.3 | 5.1 | 1.65 | 6 | nd | 1 | 124 | CR |
Absence of biliary obstruction was assessed by ultrasound. None of the patients had a history of excessive alcohol consumption (>30 g/d), and there was no evidence of exposure to hepato- or cholangio-toxic drugs. Serological tests for hepatitis B and C virus infection were negative. Metabolic liver disease including hereditary hemochromatosis, Wilson`s disease and α1-antitrypsin deficiency was excluded by appropriate biochemical tests and histologically.
Of the eleven patients with AIH alone, ten patients were designated as type 1 AIH. Antinuclear antibodies (ANA) alone were found in four patients, ANA and anti-smooth muscle antibodies (SMA) in six. Antibodies against anti-neutrophil granulocyte were present in a single patient. One patient remained autoantibody-negative. One patient with AIH had high-titer AMA without any clinical or histological evidence of PBC. Whereas all patients with AIH-PSC overlap syndrome were ANA-positive, of the patients diagnosed as having an overlap of AIH and PBC two presented AMA in serum, and a further one had PBC-specific ANA recognizing Sp100 protein (Table 1).
Clinical presentation of our patients included acute hepatitis (n = 10), with jaundice in two cases, and chronically elevated serum liver function test(s) (n = 8). Advanced liver fibrosis which was defined according to Ishak et al(6) as stages ranging from stage 4 to stage 6 was diagnosed in four patients with AIH and in two cases with overlap syndrome. The diagnosis of liver cirrhosis was made in seven patients, three with AIH and four with an overlap syndrome - [clinical stage Child A (n = 5) and Child B (n = 2)]. Four patients had concurrent immunologic diseases, including one patient each with Sharp`s syndrome, multiple sclerosis, Sjögren`s syndrome, and panniculitis.
Ten patients received BUD as first-line therapy (Table 1). Five of them had presented with acute hepatitis mimicking viral hepatitis. The median of ALT activities was 13.61 μmol/s.L (range, 10.73-38.89 μmol/s.L; reference interval 0.17-0.85 μmol/s.L). Eight patients were treated with BUD as second-line therapy (Table 1). Four of them experienced an acute exacerbation of autoimmune liver disease in spite of an immunosuppressive therapy. Exacerbation of the disease was defined as > 2x elevation of ALT activity above the ULN. Five patients treated in the second-line group were intolerant to PRD or AZA or proved to be resistant to PRD (Table 2).
| Pre-budesonidetherapy PRD(mg/d) | AZA(mg/d) | URSO(mg/d) | Diagnosis | Side effects of PRD or AZA | |
| Acute exacerbation of the disease | |||||
| B.S. | 0 | 100 | 0 | AIH | no |
| D.H. | 0 | 100 | 0 | AIH-PBC | no |
| F.C. | 0 | 100 | 0 | AIH | no |
| G.W. | 10 | 0 | 0 | AIH-PBC | no |
| No response to PRD | |||||
| S.H-D. | 40 | 0 | 1000 | AIH-PSC | Headache, hypertension |
| Side effects of the drugs | |||||
| K.C. | 10 | 100 | 0 | AIH | Weight gain of 20 kg |
| B.R. | 5 | 0 | 0 | AIH | Osteoporosis, myopathy |
| S.U. | 10 | 50 | 750 | AIH-PSC | Pancreatitis |
Liver biopsies were obtained from all patients to assess inflammatory activity and stage of fibrosis before the initiation of therapy. All biopsy specimens were fixed immediately in 10% neutralized formalin and subsequently embedded in paraffin. Deparaffinized serial sections were stained using a hematoxylin and eosin stain (H&E), periodic-acid Schiff reagent with and without diastase pretreatment, Masson`s trichrome stain, reticulin stain, and iron stain. At least eight sections (three sections stained with H&E and one section for each special stain) were evaluated per biopsy specimen. Grading and staging of hepatitis was performed according to the modified Histological Activity Index[6]. Follow-up tissue examinations were done only in patients who had a complete clinical and laboratory resolution and consented for a follow-up liver biopsy. Our protocol was approved by the local Ethics Committee of the Otto-von-Guericke University of Magdeburg. Informed consent was obtained from all patients.
BUD (Budenofalk), 3 mg thrice daily, was administered to each participant with an intention to treat for at least six months. Patients who met the criteria for a complete biochemical response and were free of BUD-related side effects were treated for an indefinite period of time. Drug intolerance, exacerbation of disease or treatment failure justified discontinuation of the medication any time. Therapy was discontinued beyond 6 mo in three patients (wk 4, wk 12 and wk 24) because of treatment failure or deterioration of disease. Patients who experienced a complete biochemical response and had also side effects of BUD or were suspected to be at risk for drug-induced side effects were given AZA in a dose of 1-1.5 mg/kg body weight. Significant cholestasis or pruritus was treated with Ursodeoxycholic Acid (Ursofalk) in a dose of 10-15 mg/kg body weight in three divided doses.
Patients were monitored at 1-mo intervals in the first three mo of therapy and at 3-mo intervals afterwards by determining serum ALT, ALP, bilirubin, and IgG levels. Treatment outcomes included clinical and biochemical remission, treatment failure, and drug toxicity or side effects. Remission was defined as absence of clinical symptoms, normal serum ALT, ALP, and IgG levels. In patients with an overlap syndrome, remission included also the near normalisation of GGT (≤ 2x ULN). Treatment failure connoted clinical and biochemical deterioration. The development of intolerable cosmetic, biochemical, and/or somatic changes during treatment indicated drug toxicity (= intolerable side effects).
Descriptive analyses were used to characterize the study population.
The serum ALT level improved significantly, and completely normalised in fifteen patients (overall response rate 83%). Before initiation of the therapy the median ALT level was 12.44 μmol/s.L (range; 1.48-28.65 μmol/s.L, reference interval 0.17-0.85). At the end of our evaluation, the median of ALT activity was 0.77 μmol/s.L (range 0.18-5.79 μmol/s.L). The median time to complete normalisation of transaminase or cholestatic liver enzyme activities was about three months. The deterioration of ALT activity was noted in a woman (TH) with advanced liver fibrosis and acute hepatitis who did not respond to BUD and had a corticosteroid-dependent AIH. Under PRD (40 mg/d) she entered a complete biochemical remission, but after reducing the doses of PRD under 20 mg she experienced again an acute exacerbation of the disease. She is now in remission receiving 150 mg AZA and 5 mg PRD. BUD therapy was ineffective in two women with liver cirrhosis after 12 and 24 wk. One of them (KM) did respond to PRD and was put on AZA after six months therapy. The other one (DC) did not respond to corticosteroid and is awaiting liver transplantation. A young man (ST) with a history of pathological elevated ALT activities, fatigue and malaise lasting for five years whose liver biopsy revealed no hepatitic changes responded rapidly to BUD leading to complete normalisation of serum liver tests and disappearance of clinical symptoms.
Levels of serum bilirubin with 5.95 mg/dL (reference interval 0.1-1.1 mg/dL), ALP with 6.62 μmol/s.L (reference interval 0.8-2.15 μmol/s.L) and IgG with 2068 mg/dL (reference interval 900-1600 mg/dL) also improved in all patients with initially pathological serum levels. The median of all these parameters (serum total bilirubin: 0.81 mg/dL, range 0.33 to 2.47; ALP: 1.41 μmol/s.L, range 0.63-2.40; IgG: 1385 mg/dL, range 928-3140) was normal at the end of our evaluation.
Side effects of BUD therapy were noted in six patients (33%). However, clinically relevant side effects including abdominal pain (n = 1), weight gain of > 3 kg (n = 3), acne (n = 2) and hair lost (n = 1) and cushingoid appearance (n = 3) were reported only in patients with liver cirrhosis. Discontinuation of the therapy was warranted only in one woman (DC) who did not respond to BUD and developed intolerable gastrointestinal symptoms. No one of the patients developed impaired glucose tolerance or overt diabetes mellitus. A man with insulin-dependent diabetes mellitus showed no deterioration of his diabetes under BUD therapy. Changes in bone mineral density were not determined during the follow-up.
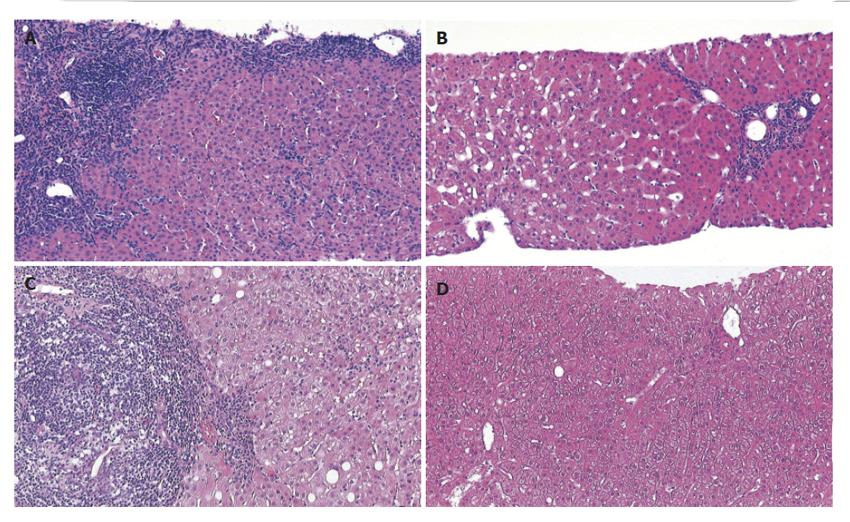
Twenty-six liver biopsies were obtained from 18 patients, of which eight were follow-up biopsies from seven patients. Histopathological changes compatible with AIH were found in eleven patients (Figure 1). Biopsy specimens from five patients showed histological signs of an overlap with either PBC or PSC (Figure 1). In a single biopsy from one patient the histological changes were very mild, with few portal tracts showing only a scattered inflammatory infiltrate, which were then categorized as unspecific changes, inconclusive for AIH. Follow-up biopsies obtained from seven patients showed a remarkable improvement of disease activity in all patients. Using the modified Ishak`s scoring system[6], disease activity was assessed with regard to periportal or periseptal interface hepatitis (maximum possible score 4), confluent necrosis (maximum possible score 6), lobular inflammation (maximum possible score 4), and portal inflammation (maximum possible score 4). By adding up the individual categories, a mean total score of 5.17 (range, 1-11) was found in the pretreatment biopsies and of 2.33 (range, 1-5) in the post-treatment biopsies (Figure 1). Staging of AIH (maximum possible score 6 - cirrhosis, probable or definite) ranged between 0 and 5. The absence of portal fibrosis (stage 0) was found in three patients, and advanced fibrosis (stage 5) was found in two. The mean values for staging showed no difference between specimens obtained before (mean value 2.39; range, 0 - 5) or after (mean value 2.10; range, 0 - 4) treatment. A mild steatosis of 20% or less was found only in four biopsy specimens of four patients. Steatosis was not found in any follow-up biopsy specimen.
BUD induced clinical and biochemical remission in the majority of our study patients with AIH including those with an overlapping PBC or PSC. Complete remission or significant improvement of liver histology was confirmed in the majority of our patients with a follow-up liver biopsy. In contrast, only three patients, two with liver cirrhosis and one with severe, corticosteroid-dependent disease, did not benefit from the therapy with BUD. 33% of patients experienced side effect(s), but only patients with advanced liver fibrosis or cirrhosis had severe adverse events as reported previously in patients with PBC because of the reduced hepatic metabolism of the drug[7]. Discontinuation of the drug was warranted only in one patient with liver cirrhosis because of drug intolerance and non-response. These findings support the value of BUD in the management of treatment-naive patients with AIH and also as salvage therapy in individuals who are intolerant to PRD or AZA or experience an acute exacerbation of the disease in spite of an immunosuppressive therapy (Table 2).
Published data with BUD in patients with AIH are limited and controversial. Currently, two papers presenting a total of 23 patients with AIH have been published in the English literature[8,9]. The first report on BUD in AIH came from Sweden. This study included 13 patients with AIH who were treated with BUD as second-line therapy, initially with a daily dose of 6 to 8 mg, after they had experienced a relapse after discontinuation of the first-line therapy with PRD and AZA. Two patients were shown to have liver cirrhosis at presentation. Using BUD, ALT level normalized within 12 wk of treatment in the majority of the study patients[8]. Further small studies including a total of 36 patients with treatment-naive AIH published as abstract from Europe suggested that BUD may induce complete biochemical remission in about two-thirds of patients after one year of therapy who received the drug as first-line therapy. The frequency of the reported drug-related side effects ranged from 29% to 55%.
In contrast, in the second published report, experiences on 10 patients receiving BUD in AIH from a single center in the U.S., suggested that BUD as second-line therapy is able to induce remission only in a minority of patients with severe steroid-dependent AIH and is inferior to PRD[9]. In this report, all patients receiving BUD experienced at least one side effect. These results may be influenced by patient selection, as the presence of fibrosis increases the risk of side effects and advanced fibrosis and steroid-dependent disease are frequently associated with treatment failure and adverse events to drugs used in AIH.
Our data indicate that BUD is able to induce not only biochemical but also histological remission in patients with treatment-naive AIH. Furthermore, BUD can also be used as salvage therapy in those cases where PRD and AZA fail or patients become intolerant to these drugs. Patients presenting with severe acute hepatitis or having an overlap syndrome with PBC or PSC experience the same frequency of remission. As advanced liver fibrosis or cirrhosis is frequently associated with side effects and treatment failure, initiation of this therapy in histologically proven liver cirrhosis may deserve further considerations.
S- Editor Guo SY L- Editor Zhang JZ E- Editor Ma WH
| 1. | Manns MP, Strassburg CP. Autoimmune hepatitis: clinical challenges. Gastroenterology. 2001;120:1502-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Czaja AJ, Bianchi FB, Carpenter HA, Krawitt EL, Lohse AW, Manns MP, McFarlane IG, Mieli-Vergani G, Toda G, Vergani D. Treatment challenges and investigational opportunities in autoimmune hepatitis. Hepatology. 2005;41:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Clissold SP, Heel RC. Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis. Drugs. 1984;28:485-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Thalen A, Brattsand R, Andersson PH. Development of glucocorticosteroids with enhanced ratio between topical and systemic effects. Acta Derm Venereol Suppl (Stockh). 1989;151:11-9; discussion 47-52. [Cited in This Article: ] |
| 5. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2003] [Cited by in F6Publishing: 1917] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 6. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3521] [Cited by in F6Publishing: 3649] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 7. | Hempfling W, Grunhage F, Dilger K, Reichel C, Beuers U, Sauerbruch T. Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis. Hepatology. 2003;38:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8:585-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Czaja AJ, Lindor KD. Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology. 2000;119:1312-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |