Published online Sep 7, 2006. doi: 10.3748/wjg.v12.i33.5344
Revised: May 28, 2006
Accepted: June 15, 2006
Published online: September 7, 2006
AIM: To evaluate peripheral blood lymphocyte subsets in patients with acute pancreatitis (AP).
METHODS: Twenty patients with mild AP (M-AP) and 15 with severe AP (S-AP) were included in our study. Peripheral blood lymphocytes were examined at d 1-3, 5, 10 and 30 by means of flow cytometry.
RESULTS: A significant depletion of circulating lymphocytes was found in AP. In the early AP, the magnitude of depletion was similar for T- and B- lymphocytes. In the late course of S-AP, B-lymphocytes were much more depleted than T-lymphocytes. At d 10, strong shift in the CD7+/CD19+ ratio implicating predominance of T- over B-lymphocytes in S-AP was found. Among T-lymphocytes, the significant depletion of the CD4+ population was observed in M-AP and S-AP, while CD8+ cells were in the normal range. Lymphocytes were found to strongly express activation markers: CD69, CD25, CD28, CD38 and CD122. Serum interleukin-2 (IL-2), IL-4, IL-5, IL-10, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) levels were significantly increased in both forms of AP. The magnitude of elevation of cytokines known to be produced by Th2 was much higher than cytokines produced by Th1 cells.
CONCLUSION: AP in humans is characterized by significant reduction of peripheral blood T- and B-lymphocytes.
- Citation: Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol 2006; 12(33): 5344-5351
- URL: https://www.wjgnet.com/1007-9327/full/v12/i33/5344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i33.5344
Excessive leukocyte activation with cytokinemia represents one of the most important mechanisms of increased mortality in early acute pancreatitis (AP). It has been hypothesized that fatal pancreatitis is a consequence of excessive leukocytic phagocyte stimulation provoked by severe trauma or persistent injury due to an agent noxious to the pancreas[1]. Lymphocytes and inflammatory mediators released by these cells are one of the most potent regulators of leukocytic phagocytes. It has been suggested that T- and B- lymphocytes activation is a key factor in the modulation of the inflammatory reaction in different diseases, including AP[2]. The role of different inflammatory mediators in AP has already been extensively studied, while the role of lymphocyte activation and its relation to disease severity in humans are still poorly understood[3-6]. Few studies have demonstrated the reduction of total peripheral lymphocytes as well as CD4+, CD8+, CD3+DR- and CD3-DR+ lymphocyte subsets in early severe AP[7-10]. In the most recent study, T cell activation has been reported in mild acute pancreatitis[11]. Another study showed that treatment with high-dose vitamin C partially restored depleted peripheral blood CD4+ cells and increased the ratio of CD4+/CD8+ cells[12]. Under the normal or pathologic conditions, CD4+ T helper (Th) lymphocytes polarize into two major subsets: Th1 and Th2. Both environmental and genetic factors act in concert to determine the Th1 and Th2 polarization. The collective data available so far are not sufficient to determine the role of specific Th cells subsets in acute pancreatitis in humans.
In this study, we, therefore, aimed to provide broad and complex evaluation of peripheral blood lymphocyte subsets in patients with AP.
Thirty five patients (16 women and 19 men; age 32-77 years, median 56 years) with AP were prospectively included into our study. In all patients, the time between the abdominal pain onset and admission to the hospital was not longer than 48 h. The control group comprised of 15 healthy volunteers (7 women and 8 men; age 19-77 years, median 41 years) without the history of recent inflammatory disease. The diagnosis was made on the basis of a history consistent with AP and serum amylase activity > 3 times the upper limit of normal range (20-90 U/L). For all the patients, additional biochemical and imaging (ultrasound and computed tomography) tests have been done to confirm the diagnosis as well as to determine the etiology and severity of AP according to Ranson’s[13] and Balthazar’s[14] criteria supplemented by serum C-reactive protein (CRP) concentration measurements. At d 2-3, CRP concentrations higher than 150 mg/L were considered as indicative of S-AP. Using these criteria, our patients were divided into mild AP (n = 20) and severe AP (n = 15) (Table 1). Among the patients with S-AP, the following systemic complications were found: pulmonary in 8 patients, circulatory in 3, renal in 3, coagulation disorders in 1 and septic complications in 1 patient. Local complications, such as acute fluid collections in 10 patients, infected necrosis in 1 and pancreatic abscess in 1 patient, were found. By etiology, 18 patients presented biliary pancreatitis, 16 alcoholic pancreatitis and 1 idiopathic pancreatitis. In the course of hospitalization, 2 patients with severe AP died. Medical University of Bialystok Ethical Committee approval had been granted for performing this study.
| Mild APn = 20 | Severe APn = 15 | PM-AP vs S-AP(Mann-Whitney U test) | |
| Etiology | |||
| Biliary | 9 (45.0%) | 9 (60.0%) | |
| Alcohol | 10 (50.0%) | 6 (40.0%) | |
| Idiopathic | 1 (5.0%) | 0 (0%) | |
| Median ranson | 1 (0-2) | 4 (2-8) | P < 0.01 |
| score (range) | |||
| Balthazar score | |||
| A | 6 (30.0%) | 0 | |
| B | 14 (70.0%) | 0 | |
| C | 0 | 2 (13.3%) | |
| D | 0 | 5 (33.3%) | |
| E | 0 | 8 (53.3%) | |
| CRP (mg/L) median (range) | |||
| D 1 | 22.9 (5.0-139.0) | 216.0 (5.0-466.1) | P < 0.01 |
| D 2 | 50.5 (5.2-135.4) | 186.2 (43.1-342.0) | P < 0.01 |
In patients with S-AP, in addition to standard treatment, prophylactic therapy with antibiotic (meropenem 500 mg tid for 10-21 d) and enteral nutrition have been implemented since the d 2-3 of the disease.
EDTA-anticoagulated blood samples were taken at admission (d 1), on d 2, 3, 5, 10 and 30. Blood samples were drawn from the antecubital vein. The absolute number of leukocytes (granulocytes, lymphocytes, monocytes) was estimated with a hematological analyzer Advia 120 (Bayer). Surface lymphocyte antigens (CD) were assayed by the direct fluorescence method for whole blood, using a flow cytometer (EPICS XL, Coulter) and double staining (FITC/PE) monoclonal antibodies (Becton Dickinson). Briefly, 100 μL samples of whole blood were incubated with 5 μL of respective monoclonal antibody solution. After 30 min of incubation in the dark at 4°C, erythrocytes were lysed, while leukocytes were fixed, stabilized (ImmunoPrep, Coulter) and analysed by EPICX XL. The use of “gate-check” [CD45-FITC/CD14-PE (BD)] allowed the division of the leukocytes population into granulocytes, lymphocytes and monocytes. Matched labelled anti-idiotype antibodies were used as negative controls (IgG1-FITC/IgG1-PE).
Populations and subpopulations of lymphocytes were evaluated using the following differentiation antigens: CD19-/CD7+ (T-lymphocytes), CD3+/CD4+ (Th- lymphocytes), CD3+/CD8+ (cytotoxic T-lymphocytes) and CD19+/CD7- (B-lymphocytes).
The following CD antigens were examined: CD3/CD69, CD7/CD122 and CD8/CD38, CD8/CD28.
CD19/CD122 expression was determined in B-lympho-cytes.
Cell preparation: Mononuclear cells were isolated by density gradient concentration on Histopaque 1077 (Sigma). Highly purified mononuclear cells (about 98%, by Advia 120) were obtained. The cells were subsequently suspended in tubes with phosphate-buffered saline.
Determination of spontaneous apoptosis: Apoptosis was determined using Annexin V-Fluos and propidium iodide double staining. Apoptosis is accompanied by a loss of membrane phospholipid asymmetry, resulting in the exposure of phosphatidylserine at the surface of the cell. Quantitative measurement of phosphatidylserine exposure was possible using the binding of fluorescein isothiocyanate-labelled annexin V to phosphatidylserine. For the annexin V assay, cells were incubated for 1 h at 37°C in 2 mL of buffer containing FITC-labelled annexin V and propidium iodide (PI) and line specific markers (CD3, CD19, CD33, CD14, PE). Flow cytometric analysis was performed by Coulter EPICS XL.
Determination of stimulated apoptosis was carried out as previously described[15]. Isolated lymphocytes were incubated in six-well plates (Falcon, Becton Dickinson) with RPMI 1640 medium (GIBCO) without fetal bovine serum at 37°C in a humidified atmosphere containing 50 mL/L CO2. After 24 h of incubation, cells were stained with a dye mixture (10 μmol/L acridine orange and 10 μmol/L ethidium bromide; Sigma) prepared in phosphate-buffered saline (PBS). Two-hundred cells per sample were examined by fluorescence microscopy, and the cells were characterized according to the following criteria: (1) native cells-fine reticular pattern of green stain chromatin; (2) necrotic cells-bright orange stain chromatin; and (3) apoptotic cells-green stain chromatin which was highly condensed and uniformly stained by acridine orange.
The BD Human Th1/Th2 Cytokine CBA kit was used to measure IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ levels in serum samples. The samples diluted with the appropriate volume of Assay Diluent were transferred into the assay tubes containing capture beads (covered with respective cytokine antibody) and PE detection reagent. The standard curve for each cytokine was defined for concentrations from 20-5000 ng/L. The assay was performed using a FACSCalibur (BD) flow cytometer.
Statistical analysis was performed using the non-parametric Mann-Whitney’s U test. P values less than 0.05 were considered statistically significant. Results were expressed as mean ± SD.
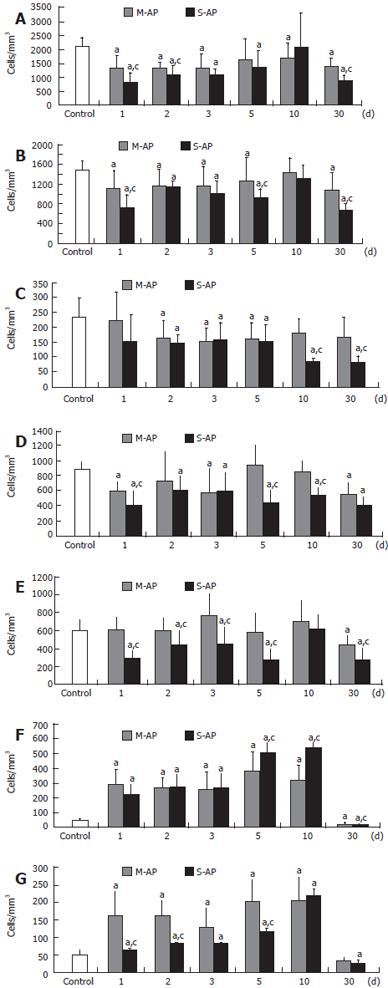
At d 1, significant decrease of total lymphocyte number to 63% and 39% of the healthy control was found in patients with M-AP and S-AP, respectively (Figure 1A). In the course of the disease, lymphocyte number gradually increased, reaching 79% and 97% of the control in M-AP and S-AP at d 10, respectively. At d 30, lymphocyte number fell again to 65% and 42% of the control, respectively, in M-AP and S-AP. Analysis of CD7 and CD19 molecule expression showed that T-lymphocyte (CD7+) population was especially reduced in the early course of S-AP (Figure 1B). Thereafter, it was constantly increasing, returned to normal at d 10 and decreased again to 46% of the control at d 30. In M-AP, depletion of T-lymphocyte population was less evident (Figure 1B). Some reduction of B-lymphocyte (CD19+) number was found in the early S-AP, while marked depletion to 36% and 34% of the control was noticed at d 10 and 30, respectively (Figure 1C). In M-AP, the decrease of B-lymphocyte number was not so persistent.
Two major subsets of T-lymphocytes are represented by CD4+ and CD8+ cells, also known as T4-helper cells and T8-cells or cytotoxic T-lymphocytes (CTLs), respectively. At d 1, the number of circulating CD4+ cells significantly dropped in both M-AP and S-AP to 68% and 45% of the control, respectively (Figure 1D). In M-AP, at d 5 and 10, it returned to the range of normal level, while in S-AP it remained significantly depleted until d 30. The number of circulating CD8+ cells remained in the range of the normal level in the early M-AP, including the d 10 (Figure 1E). At d 30, it decreased to 73% of the control. At d 1 of S-AP, CD8+ cells were markedly depleted to 47% of the control and remained below normal level until d 5. CD8+ returned to normal level at d 10, and depleted again to 47% of the control at d 30.
In patients with both M-AP and S-AP, some fluctuations of lymphocyte T/B (CD7+/CD19+) ratio were observed (Table 2). At d 10, the ratio was markedly increased because the T-lymphocyte number was close to normal and the significantly diminished B-lymphocyte level. In M-AP and S-AP, the lowest CD4+/CD8+ cells ratio was found at d 3 and 10, respectively (Table 2).
| Control | Days | |||||||
| 1 | 2 | 3 | 5 | 10 | 30 | |||
| CD7+/CD19+ | M-AP | 6.4 ± 1.9 | 4.9 ± 1.2a | 7.0 ± 2.3 | 7.6 ± 2.1 | 7.8 ± 2.1 | 7.9 ± 1.9 | 6.5 ± 1.7 |
| S-AP | 4.6 ± 1.2a | 8.0± 2.1a | 6.3 ± 1.9 | 6.1 ± 1.8 | 15.8 ± 4.7ac | 8.7 ± 2.0ac | ||
| CD4+/CD8+ | M-AP | 1.5 ± 0.6 | 1.0 ± 0.4a | 1.2 ± 0.5 | 0.7 ± 0.6a | 1.6 ± 0.5 | 1.2 ± 0.5 | 1.2 ± 0.5 |
| S-AP | 1.4 ± 0.5c | 1.4 ± 0.6 | 1.3 ± 0.6c | 1.6 ± 0.6 | 0.9 ± 0.6a | 1.5 ± 0.5 | ||
Peripheral blood lymphocytes in both M-AP and S-AP patients showed a dramatic increase of intracellular signaling manifested by CD69 expression. At d 1, it was elevated 6.0-fold and 4.6-fold in M-AP and S-AP, respectively (Figure 1F). At d 10, CD69 expression in peripheral blood lymphocytes reached a maximum (11.2-fold over the control level) in S-AP and then dramatically declined to 52% and 40% of the healthy control in M-AP and S-AP, respectively.
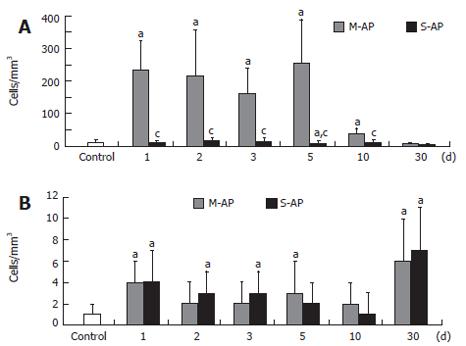
CD25/IL-2Rα expression, known as the lymphocyte proliferation marker, was elevated especially in M-AP (Figure 1G). At d 1 and 10, it increased to 3.3-fold and 4.2-fold over the control, respectively. In S-AP, a marked elevation of CD25/IL-2Rα expressing lymphocytes (4.5-fold over the control) was found at the d 10. At d 30, the number of the lymphocyte subpopulation significantly decreased to 63% in M-AP and to 47% in S-AP. The number of peripheral blood T-lymphocytes expressing CD122 sharply increased in patients with M-AP (Figure 2A). At d 1, it increased almost 20-fold compared to the control, and remained highly elevated until d 5. At the d 10, the number of lymphocytes significantly diminished and at d 30, reached 50% of the control level. In the course of S-AP, the number of T-lymphocytes expressing CD122 did not significantly change. However, at d 30, it dropped to 33% of the normal level.
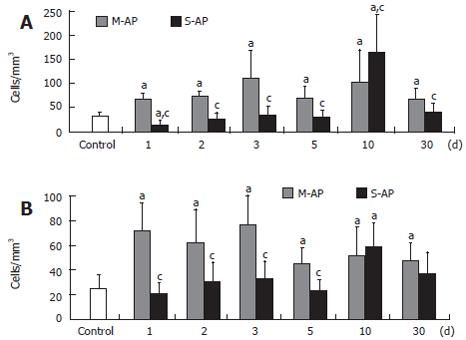
The number of peripheral blood B-lymphocytes with the same marker of activation was significantly elevated in both M-AP and S-AP patients (Figure 2B). Interestingly, concerning CD122 expression, the highest level of B-lymphocyte activation, 6- and 7-fold were found in M-AP and S-AP at the d 30, respectively. Figure 3 shows the pattern of activation of T-lymphocyte CD8+ subset. In M-AP, the number of cells expressing CD38 increased to 20.6% of the control level at d 1, reached the maximum level of 33.9% at d 3 and remained elevated at 20.3% at d 30 (Figure 3A). In S-AP, the opposite trend was noted with significant diminishing of CD8+CD38+ lymphocytes to 45% of the control at d 1. It returned to normal level at d 3, while a strong temporary elevation to 49.7% of the control at d 10 was noted.
Concerning CD28 expression, CD8+ T-lymphocyte activation was the strongest observed in M-AP patients (Figure 3B). At d 1, it reached 28.8% of the control, remained at the similar level until d 3 and then slightly decreased to 19.2% of the control at d 30. In S-AP, it oscillated around normal level until d 5, increased to 23.6% of the control at d 10, and remained slightly elevated at d 30.
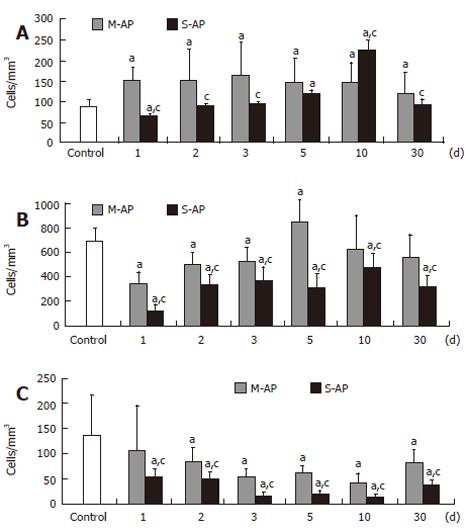
Expression of CD95/Fas-R/Apo-1 receptor protein, which plays an important role in apoptosis initiation, was enhanced in early M-AP and reached a maximum (18.8% of the control) at d 3 (Figure 4A). It remained slightly elevated throughout the course of the disease. In S-AP, the number of lymphocytes expressing CD95/Fas-R/Apo-1 slightly decreased to 7.6% of the control at the d 1 and then constantly raised up to 26.8% at d 10. Thereafter, it returned to the control level at the d 30.
At d 1, the number of peripheral blood T-lymphocytes that stained for annexin V (AnnV+CD3+) significantly decreased in M-AP and S-AP to 49% and 17% of the normal level, respectively (Figure 4B). Thereafter, it gradually increased, and in M-AP slightly exceeded normal level (12.2% of control) at d 5. In S-AP, the number of AnnV+CD3+ lymphocytes remained diminished during the course of whole observation. The number of peripheral blood B-lymphocytes that stained for annexin V (AnnV+CD19+) started to decrease at d 1 in both forms of AP, and at d 10, reached the minimum value, i.e. 30% and 10% of the normal level in M-AP and S-AP, respectively (Figure 4C). Table 3 shows the viability of lymphocytes isolated from peripheral blood of patients with pancreatitis. In both forms of pancreatitis, in non-stimulated cells, the rates of apoptosis and necrosis were at similar levels as in the control. Stimulated cells from the healthy control died predominantly by necrosis. However, in stimulated cells of patients with pancreatitis, lymphocytes died predominantly by apoptosis, without showing any significant difference between M-AP and S-AP.
We measured serum concentrations of cytokines produced by Th1 and Th2 lymphocytes in the first 10 days of the disease (Tables 4, 5). IL-2, IFN-γ and TNF-α represent the pattern characteristic for Th1, while IL-4, IL-5 and IL-10 are produced by Th2. IL-2 and IFN-γ levels were much higher in S-AP than in M-AP, while TNF-α was slightly elevated in both forms of pancreatitis (Table 4). At d 1, we observed a huge increase by 27-fold and 33-fold in the IL-4 level in M-AP and S-AP, respectively (Table 5). It remained highly elevated in the whole early course of pancreatitis. IL-5 was significantly increased in both forms of pancreatitis during the first 5 d of observation. However, it returned to normal level at d 10. A remarkable increase of IL-10 was noted in both forms of pancreatitis. The peak, 36-fold and 44-fold elevation, was observed in M-AP and S-AP at d 2, respectively (Table 5).
| Control | Days | ||||||
| 1 | 2 | 3 | 5 | 10 | |||
| IL-2 | 6.2 ± 5.4 | M-AP | 20 ± 6a | 18 ± 8a | 18 ± 7a | 12 ± 7a | 8 ± 4a |
| (ng/L) | S-AP | 21 ± 10a | 24 ± 11ac | 25 ± 11ac | 13 ± 9a | 9 ± 5a | |
| IFN-γ | 6.0 ± 5.7 | M-AP | 45 ± 24a | 44 ± 25a | 41 ± 14a | 28 ± 11a | 17 ± 5a |
| (ng/L) | S-AP | 258 ± 24ac | 49 ± 23a | 40 ± 19a | 25 ± 10a | 16 ± 6a | |
| TNF-α | 1.8 ± 2.4 | M-AP | 2.9 ± 1.2a | 2.9 ± 1.6a | 1.5 ± 0.9 | 0.9 ± 0.7 a | 1.0 ± 0.9a |
| (ng/L) | S-AP | 3.2 ± 1.4a | 3.2 ± 1.5a | 1.0 ± 1.0ac | 1.0 ± 0.7a | 1.1 ± 0.9a | |
| Control | Days | ||||||
| 1 | 2 | 3 | 5 | 10 | |||
| IL-4 | 6.0 ± 5.8 | M-AP | 164 ± 57a | 133 ± 60a | 79 ± 34a | 82 ± 43a | 60 ± 33a |
| (ng/L) | S-AP | 198 ± 74ac | 175 ± 68ac | 92 ± 42ac | 94 ± 45ac | 72 ± 35ac | |
| IL-5 | 5.2 ± 4.9 | M-AP | 19 ± 9a | 29 ± 9a | 17 ± 7a | 52 ± 23ac | 4 ± 3 |
| (ng/L) | S-AP | 23 ± 7a | 40 ± 12ac | 18 ± 7a | 39 ± 19ac | 3 ± 3 | |
| IL-10 | 1.4 ± 1.6 | M-AP | 40 ± 27a | 50 ± 33a | 49 ± 27a | 32 ± 19a | 39 ± 23a |
| (ng/L) | S-AP | 52 ± 36ac | 62 ± 36ac | 52 ± 36a | 50 ± 36ac | 47 ± 31a | |
Alteration of the immune system is one of the major mechanisms responsible for early and late mortality in severe AP. Excessive inflammatory reaction, known as systemic inflammatory response syndrome (SIRS), is considered as the leading cause of death in early AP[4-6]. The role of lymphocytes in this phenomenon has been partly studied and the knowledge of the mechanisms in humans is still incomplete[7-11,16]. Our study provides, probably for the first time, a broad and complex analysis of peripheral blood lymphocyte subsets with reference to different time points and different severity forms of acute pancreatitis. In agreement with the previous studies[7-11,16], we also found a significant depletion of circulating lymphocytes which was much more profound in the severe form of pancreatitis. In the early period of 1-5 d of the disease, the magnitude of peripheral depletion was similar for T- (CD7+) and B- (CD19+) lymphocytes. However, in the second week and at d 30 of S-AP, B-lymphocytes were found to be particularly depleted. Concerning the relations between these subsets of lymphocytes, the middle of the second week (d 10) was critical because of the strong predominance of T- over B-lymphocytes in S-AP. Depletion of the CD19+ lymphocyte subset in early acute pancreatitis has also been recently reported[16]. Among T-lymphocytes, at d 1 and 3 of M-AP as well as at d 10 of S-AP, we observed significant depletion of the CD4+ population, while CD8+ cells were in the normal range. It created a temporary imbalance in the cell ratio with the apparent prevalence of CD8+ over the CD4+ cells. CD8+ cells are known as cytotoxic T-lymphocytes (CTL) and some of them differentiate into T8-suppressor cells. Significant depletion of peripheral blood CD4+ and CD8+ subpopulations of T-lymphocytes in the course of acute pancreatitis has previously been reported[7-9,12,16]. However, observations made by the others were restricted mostly to one or up to three different time periods of pancreatitis.
The spectrum of cytokines known to modulate lymphocyte differentiation and function (IL-2, IFN-γ, TNF-α, IL-4, IL-5 and IL-10), evaluated in the early course of pancreatitis, showed all of them significantly increased in both forms of AP, however, the highest values were observed in S-AP. Previous studies showed the elevated IL-2, TNF-α and IL-10 levels in human AP[11,12,17,18]. To our knowledge, no data are available in literature on serum levels of IFN-γ, IL-4 and IL-5 in human AP. In our study, the magnitude of elevation of cytokines known to be produced by Th2 cells, namely IL-4, IL-5 and IL-10, was much higher than cytokines produced by Th1 cells, represented by IL-2 and IFN-γ. This finding suggests that in the course of AP, Th1 subpopulation of CD4+ cells is suppressed more strongly than Th2. Early IL-4 expression during an immune response is critical for determining the development of Th2 cells[19]. Cytokines produced by Th2 cells enable activated B-lymphocytes to proliferate, stimulate activated B-lymphocytes to synthesize and secrete antibodies, promote the differentiation of B-lymphocytes into antibody-secreting plasma cells, and enable antibody-producing cells to switch the class of antibodies being produced[20]. In our study, B-lymphocyte activation was the strongest at d 30, while T-lymphocyte activation remained normal or even below the control level. This pattern of lymphocyte activation might be the consequence of shift from Th1 to Th2 in patients with AP. Th1 and Th2 cells represent polarized forms of the CD4+ Th cell-mediated immune response. Th1 cells produce IL-2, IFN-γ and TNF-α which cooperate with B-cells for the production of antibodies, activate phagocytic cells and CD8+ T-cells, thus promoting cell-mediated immunity and cytotoxic T-cell responses[21]. In contrast, cytokines released by Th2 cells (IL-4, IL-5, IL-9 and IL-13) induce B-cells to produce high amounts of IgG4 and IgE in humans, promote the differentiation and growth of mast cells and eosinophils, and inhibit several phagocytic functions[21]. Of note, while IL-4 inhibits the development of Th1 cells, IFN-γ inhibits the development of Th2 cells[21]. Treg cells are a highly heterogeneous family, which includes type 3 Th (Th3) cells, T regulatory 1 (Tr1) cells, and CD4+ CD25+ T cells. Interestingly, Tr1 cells are mainly able to produce IL-10. Therefore, the huge elevation of serum IL-10 level found in our study may suggest that Tr1 cells are especially active in the course of AP.
In our study, significant activation of lymphocytes was observed, which was shown by strong expression of CD69, CD25, CD28, CD38 and CD122 on T-lymphocytes as well as CD122 on B-lymphocytes. The surface receptors CD69 and the IL-2 receptor (CD25) are early markers of activation. Increased expression of CD69 as well as CD25 on CD3+, CD4+ and CD8+ cells has recently been reported in mild AP[11]. It has also been reported that the number of B-lymphocytes (CD19+) expressing CD69+ was significantly lower in patients with severe pancreatitis than in patients with mild pancreatitis[16]. Consequently, the conclusion has been made that patients with severe pancreatitis show impaired early activation of peripheral CD19+ cells. Interestingly, in our study, evaluation of CD19+ cells expressing CD122, which is another marker of lymphocyte activation, showed significant activation of the B-lymphocyte subset bearing this marker. These observations may suggest that the CD19+ cell subset is heterogeneous and the subsets of these cells react in a different manner in the course of pancreatitis.
Marked activation of different subsets of lymphocytes may explain why we have observed strong elevation of different cytokines in peripheral blood despite a significant decrease in the number of circulating lymphocytes. The question remains, however, what was the reason for significant depletion of peripheral blood lymphocytes in pancreatitis. One of the possible reasons is strong migration of activated lymphocytes to the site of inflammation, including the pancreas and other tissues like lungs or kidneys, as a part of SIRS[22]. Another possible explanation is an excessive elimination of lymphocytes by apoptosis. Expression of CD95, known as Apo-1, Fas or death receptor on lymphocytes was increased in early M-AP and in S-AP at d 10. Surprisingly, the expression of annexin V, which is a marker of ongoing apoptosis, was decreased in early and late S-AP on both T- and B-lymphocytes. In the late period of M-AP, annexin V expression on T-lymphocytes was in the normal range, while it was decreased on B-lymphocytes. This finding is in agreement with our observation of increased activation of B-lymphocytes in the late course of pancreatitis. It is known that stimulation by factors inducing cell proliferation may inhibit apoptosis[23]. This phenomenon is the basis of the method evaluating cell susceptibility to apoptosis[15]. Using this in vitro method, we found that lymphocytes from patients with pancreatitis were primed to apoptosis. Similar results of in vitro incubation of lymphocytes from patients with S-AP have previously been reported[10]. Taken together, peripheral blood lymphocyte depletion in acute pancreatitis may result from both excessive apoptosis and migration to the site of inflammation.
Tissue damage caused not only by severe AP but also by traumatic incidents, like severe burns, accident trauma, major surgical interventions or sepsis, induces commensurate with the severity of damage (damage load), genetic factors (gene polymorphism), the general condition of the host and the type of antigens (antigenic load), both local and systemic release of pro-inflammatory cytokines and phospholipids[24-26]. Polymorphonuclear leukocytes, monocytes, tissue macrophages, lymphocytes, natural killer cells, and parenchymal cells are involved in a complex network of the host defense response. An overwhelming pro-inflammatory response (hyper-inflammation) leads to the clinical manifestations of SIRS and finally to host defense failure expressed by multiple organ dysfunction syndrome (MODS) or multiple organ failure (MOF). The up-regulation of pro-inflammatory factors, such as TNF, IL-1 and IL-6, observed during the SIRS phase can be followed by a second response that involves down-regulation of IFN-γ and increase in anti-inflammatory cytokines, such as IL-10 and transforming growth factor-β. This counter-regulatory phenomenon is called the compensatory anti-inflammatory response syndrome (CARS)[24]. Aside from cytokine profiles, critical injury and sepsis are also correlated with dysfunction of many immune cells. After severe injury, CD4+ T-cell differentiation into Th phenotypes is altered and there is an early expression of Th1 cytokines (IL-12, IFN-γ), followed 24 to 72 h later by a predominance of the Th2 cytokine (IL-4) and depression in the production of IL-2 and IFN-γ[24]. It has been found that severe AP, burns, accident trauma, major surgical interventions or sepsis are associated with a significant decrease in total systemic lymphocyte counts, including both CD4+ and CD8+ cells. The development of immunosuppression in subjects suffering from traumatic events is often associated with elevation of IL-10 and the shift of the Th1/Th2 balance towards a Th2 response[24,26]. The majority of data about lymphopenia in trauma-related cellular immune defects refer to T-cells with scant information about B-cells. Lung and/or kidney failure may take place in the course of specific diseases affecting these organs or may be a part of MOF or MODS resulting from tissue damaging events, including S-AP. It has been found that peripheral blood B-cells, but not CD4+ and CD8+, were significantly lower in uremic patients. This phenomenon may be partially attributed to an increased susceptibility to apoptosis associated with a decreased expression of Bcl-2[27]. On the contrary, T lymphopenia with normal counts of peripheral blood B-cells has recently been reported in end-stage renal disease[28,29]. Ex vivo evaluation of T-cells showed an increased number of annexin V and CD95 (Fas)-positive T-cells, suggesting that apoptosis may be responsible for excessive elimination of this lymphocyte subpopulation[28]. A progressive decrease in renal function associated with activation and selective loss of naïve CD4+ and CD8+ T-cells as well as CD4+ central memory cells has recently been reported. These changes may contribute to the clinical phenomena of the uremia-associated immune defect in patients with chronic renal disease[29]. Acute respiratory distress syndrome (ARDS), a sudden, life-threatening lung failure, can complicate the course of severe AP and other diseases with critical tissue damage. Severe lymphopenia has recently been reported in patients with severe acute respiratory syndrome (SARS) who developed ARDS[30]. ARDS occurring in renal transplantation patients with pneumonia is accompanied by a significant decrease in blood CD4+ and CD8+ T cells. In these patients, the recovery of the disease met the recovery course of their immune system[31]. In conclusion, the available data indicate that lymphopenia caused by the decrease of different lymphocyte subpopulations is characterized not only for severe AP but also for other diseases with severe tissue damage. However, with the exception of our study, the available data provide mostly the information about a single or just a few representatives of lymphocyte subpopulations in a specific disease. Further studies involving broad spectra of immune processes regulation are necessary in order to better understand the complex phenomenon of immunity disorders in diseases with critical tissue damage.
In summary and conclusion, in patients with acute pancreatitis, we found a significant depletion of circulating lymphocytes which was much more profound in the severe form of the disease. In the early period of pancreatitis, the magnitude of peripheral depletion was similar for T- and B- lymphocytes. However, in the late course of S-AP, B-lymphocytes were particularly depleted. The second week of the disease seems to be critical for the cellular immunity function, because of the strong shift in the CD7+/CD19+ ratio implicating predominance of T- over the B-lymphocytes in S-AP. Among T-lymphocytes, the significant depletion of CD4+ population was observed in M-AP and S-AP, while CD8+ cells were in the normal range. It created a temporary imbalance in the cell ratio with the apparent prevalence of CD8+ over the CD4+ cells. Serum IL-2, IFN-γ, TNF-α, IL-4, IL-5 and IL-10 levels were significantly increased in both forms of AP, with the highest values found in S-AP. The magnitude of elevation of cytokines known to be produced by Th2 cells was much higher than cytokines produced by Th1 cells. This finding suggests that in the course of AP, Th1 subpopulation of CD4+ cells is suppressed more strongly than Th2. The pattern of lymphocyte activation we found in AP patients may be the consequence of shift from Th1 to Th2. Strong elevation of serum level of different cytokines, despite a significant decrease in the number of circulating lymphocytes, may be explained by the significant activation of T- as well as B-lymphocytes. Peripheral blood lymphocyte depletion in acute pancreatitis may result from both excessive apoptosis and migration to the site of inflammation. The data obtained in this study systematize our current knowledge on different lymphocyte subsets in acute pancreatitis and show avenues for future research on cellular immunity in this disease.
S- Editor Pan BR L- Editor Kumar M E- Editor Ma WH
| 1. | Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation. Int J Pancreatol. 1988;3:105-112. [PubMed] [Cited in This Article: ] |
| 2. | Mora A, Pérez-Mateo M, Viedma JA, Carballo F, Sánchez-Payá J, Liras G. Activation of cellular immune response in acute pancreatitis. Gut. 1997;40:794-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 501] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Ogawa M. Acute pancreatitis and cytokines: "second attack" by septic complication leads to organ failure. Pancreas. 1998;16:312-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 25] [Reference Citation Analysis (0)] |
| 7. | Curley PJ, McMahon MJ, Lancaster F, Banks RE, Barclay GR, Shefta J, Boylston AW, Whicher JT. Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: relationship to endotoxin, interleukin 6 and disease severity. Br J Surg. 1993;80:1312-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Widdison AL, Cunningham S. Immune function early in acute pancreatitis. Br J Surg. 1996;83:633-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Pezzilli R, Billi P, Beltrandi E, Maldini M, Mancini R, Morselli Labate AM, Miglioli M. Circulating lymphocyte subsets in human acute pancreatitis. Pancreas. 1995;11:95-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Takeyama Y, Takas K, Ueda T, Hori Y, Goshima M, Kuroda Y. Peripheral lymphocyte reduction in severe acute pancreatitis is caused by apoptotic cell death. J Gastrointest Surg. 2000;4:379-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Sweeney KJ, Kell MR, Coates C, Murphy T, Reynolds JV. Serum antigen(s) drive the proinflammatory T cell response in acute pancreatitis. Br J Surg. 2003;90:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Du WD, Yuan ZR, Sun J, Tang JX, Cheng AQ, Shen DM, Huang CJ, Song XH, Yu XF, Zheng SB. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003;9:2565-2569. [PubMed] [Cited in This Article: ] |
| 13. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81. [PubMed] [Cited in This Article: ] |
| 14. | Balthazar EJ. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am. 1989;27:19-37. [PubMed] [Cited in This Article: ] |
| 15. | Dabrowska MI, Becks LL, Lelli JL Jr, Levee MG, Hinshaw DB. Sulfur mustard induces apoptosis and necrosis in endothelial cells. Toxicol Appl Pharmacol. 1996;141:568-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Pezzilli R, Maldini M, Morselli-Labate AM, Barakat B, Romboli E, Beltrandi E, Migliori M, Tomassetti P, Corinaldesi R. Early activation of peripheral lymphocytes in human acute pancreatitis. J Clin Gastroenterol. 2003;36:360-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Early prediction of organ failure by combined markers in patients with acute pancreatitis. Br J Surg. 2005;92:68-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol. 2000;85:9-18; quiz 18, 21. [PubMed] [Cited in This Article: ] |
| 20. | Jelinek DF. Regulation of B lymphocyte differentiation. Ann Allergy Asthma Immunol. 2000;84:375-85; quiz 385-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both. Immunology. 2004;112:352-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 301] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 359] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653-2659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 24. | Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006;21:160-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 550] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 26. | Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression. Langenbecks Arch Surg. 2004;389:475-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Fernández-Fresnedo G, Ramos MA, González-Pardo MC, de Francisco AL, López-Hoyos M, Arias M. B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrol Dial Transplant. 2000;15:502-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204-212. [PubMed] [Cited in This Article: ] |
| 29. | Litjens NH, van Druningen CJ, Betjes MG. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol. 2006;118:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Chen CY, Lee CH, Liu CY, Wang JH, Wang LM, Perng RP. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc. 2005;68:4-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Sun Q, Liu ZH, Chen J, Ji S, Tang Z, Cheng Z, Ji D, Li LS. An aggressive systematic strategy for acute respiratory distress syndrome caused by severe pneumonia after renal transplantation. Transpl Int. 2006;19:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |