INTRODUCTION
Achalasia is a rare motility disorder of the esophagus characterized functionally and manometrically by failed relaxation of a hypertensive lower esophageal sphincter and the absence of progressive peristalsis of the esophageal body[1]. The predominant morphologic alteration is a reduction in or complete absence of ganglia cells in the myenteric plexus in combination with an inflammatory infiltrate[2-5]. Patients with achalasia are at an increased risk of developing squamous cell carcinoma of the esophagus. The incidence of carcinoma reported in the literature ranges from 0%-29%, and has led various authors to describe achalasia as a precancerous stage[6-12]. In achalasia, the carcinoma is frequently, presumably due to the presence of dysphagia, diagnosed only at an advanced stage, with a corresponding poor prognosis[8,13,14]. The detection of precancerous alterations thus remains a diagnostic challenge. In addition to the currently available optical techniques, static DNA image cytometry offers the possibility of identifying cytogenetic alterations at an early stage. In the present study, we therefore aimed to determine and investigate DNA aneuploidy in biopsy specimens from patients with primary achalasia, using a rapid diagnostic test.
MATERIALS AND METHODS
Patients
From November 1, 2003 to October 7, 2004, specimens were collected (forceps biopsies) from the middle third of the esophagus in 15 patients with achalasia in the course of screening endoscopy. The study was performed with approval of the Local Ethics Committee [number 837.347.03 (4009), 2003]. The diagnosis was confirmed in all patients enrolled in the study by esophageal manometry, as well as endoscopic and radiological examination, which enabled exclusion of the presence of secondary achalasia. Enrolled in the study were 9 male and 6 female patients with a median age of 49 (28-75) years at the time of biopsy. The median interval from the time of the initial diagnosis was 54 (1-647) mo.
Squamous cell carcinoma of the esophagus
In 15 patients with squamous cell carcinoma of the esophagus, reference specimens were obtained from the center of the carcinoma, as well as from peritumoral tissue 2 cm proximal to the tumor margin (surplus material from the surgical specimen resected in the course of esophagectomy with informed patient consent). Included in this group of patients were 1 female and 14 male patients with a median age of 64 (40-79) years.
Clinical symptoms
Clinical symptoms were assessed with the Eckardt symptom score[15] . The possible score for each symptom ranged from 0-3, depending upon the presence of dysphagia, regurgitation, and retrosternal pain occurring occasionally, daily, several times a day, or at each mealtime. An additional score was recorded for weight loss (no weight loss, < 5 kg, 5-10 kg, and >10 kg). The total possible score therefore ranged from a minimum of 0 to a maximum of 12 points.
Esophageal manometry
The investigations were carried out using a capillary perfusion system as previously described[15] . Recorded in addition to the resting tonus and relaxation of the lower esophageal sphincter were the contraction amplitudes at 5 and 10 cm above the lower esophageal sphincter after 10 barium swallows. The manometric criteria for the diagnosis of achalasia included the absence of peristalsis in the esophageal body, as well as a non-relaxing lower esophageal sphincter. Due to the fact that in one patient passage of the manometry probe into the lower esophageal sphincter was not possible, and another patient had previously undergone distal esophagus and fundus resection at an outside institution, the documentation in these patients refers to the motility of the esophageal body only.
Radiological examination
Barium swallow was performed in the patient in the supine, semisupine, and upright positions. Measurements were made of the maximum diameter of the esophageal body, as well as of the narrowest point of the gastroesophageal junction.
Therapy
Pneumatic dilatations were carried out in a total number of 10 patients (3 patients had a second, and 1 patient a third dilatation in the course of therapy). Five patients had a prior Heller myotomy in combination with anterior partial fundoplication according to Dor et al[16]. Nine additional patients were scheduled to undergo myotomy (in 1 of these patients re-myotomy was performed for recurrent disease).
Histology
The paraffin-embedded specimens were cut into 3-4-μm thick sections and stained with hematoxylin and eosin (H&E). The non-malignant tissue was classified as normal, inflammatorily altered, or dysplastic. The definition of invasive carcinoma was based on the detection of infiltrative growth patterns for individual malignant cells or glands.
Immunohistochemistry
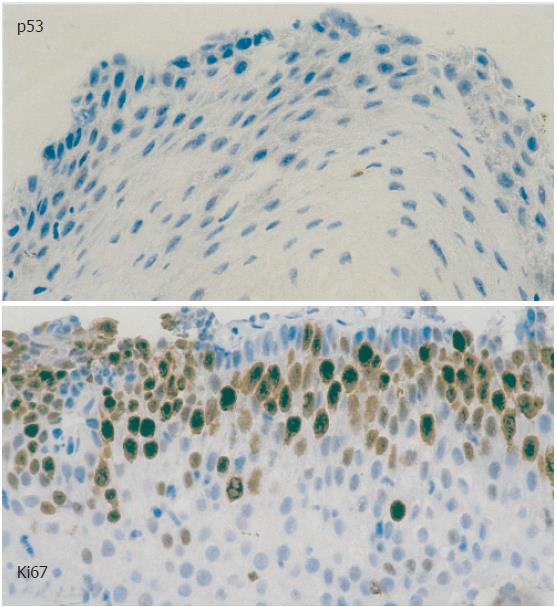
Consecutive sections for immunohistochemical evaluation were cut after deparaffinization and rehydration, and 30 mL/L hydrogen peroxide was used to block endogenous peroxidase. The slides were incubated with the monoclonal antibodies MIB-1 (mAb) (1:200, Dako, Denmark) for Ki67 antigen and PAb 1801 (1:100, Becton Dickinson) for p53. After microwave pretreatment, specimens were incubated with MIB-1 and PAb for 1 h at room temperature, followed by sequential 20-min incubations with biotinylated anti-mouse IgG and peroxidase-labeled streptavidin. The staining procedure was described as positive if more than 20% of cells reacted for p53-mAb.
Image cytometric DNA analysis
A computerized image analysis system was used for DNA measurements (AutoCyte QUIC-DNA Workstation; AutoCyte, Elon College, NC, USA). The measurements were analyzed with AutoCyte QUIC-DNA software. The homogenized tissue was dissolved in 2 mL 0.01 g/L pronase solution (Pronase (lyophilyzed) Dako, Denmark) and incubated in PBS (pH 7.4). The prepared tissue was then filtered through a 50-μL pore size nylon mesh. This method enables specific staining of DNA in situ[17] . The possibility of DNA quantification according to the Feulgen reaction is due to the fact that the staining intensity during this reaction is in direct proportion to the DNA concentration[18] .
Approximately 250-300 test cells were measured for interactive image cytometry. The cells were selected on the screen on the basis of nuclear morphology. The DNA content of >30 reference nuclei was measured in each case to obtain accurate values at the different staining intensities for the individual slides. A reference extinction was assigned to the normal 2c-value of the reference cells. Gray levels in the microscopic image were transformed into digitalized signals and evaluated, with the System 256 allowing differentiation between gray level intervals. Measurements were made with 566-nm monochromic light. Light absorption is proportional to the DNA content of the cell. DNA aneuploidy is assumed when cells with a DNA content of 2xG1 of the reference cell fraction are detected. In tissues without polyploidization, a threshold value of the 5c-results (5c-exceeding events) is sufficient for classification. The demonstration of 1% of cell nuclei with a DNA content of > 5c serves as proof of malignancy[19]. The following indices were calculated based on the DNA measurements: Aneuploidy (measured values outside the interval of +/- 12.5% of the value of the simple powers of 2c); aneuploid rate (percentage of aneuploid cells out of the total number of test cells); grading of malignancy according to Boecking (logarithmic transformation of the variance of DNA values on a scale from 0-3); and the 5c-, 7c- and 9c-exceeding rate (percentage of cells with ploidy values higher than 5c, 7c and 9c in relation to the total number of test cells).
Statistical analysis
The SPSS 11.0 software package (SPSS, Chicago, IL, USA: 2001) was used for data analysis. Data were expressed as median values with ranges (minimum-maximum) and as percentages. In the comparative analysis of the patient groups, the χ2 test with Pearson’s correction, as well as Fisher’s exact test were employed. The Kruskal-Wallis test was used for the comparison of DNA alterations in achalasia, peritumoral, and carcinoma biopsies. Spearman’s linear regression model served to calculate potential correlations between mucosal DNA alterations or proliferation markers and clinical findings. P <0.05 was considered statistically significant.
RESULTS
The median symptom score according to Eckardt was 3 (range 1-10) points at the time of the investigation. Roentgenographic findings showed a mean maximum esophageal body diameter of 30 (20-60) mm, and a mean lumen diameter of 7 (1-10) mm at the narrowest point of the esophagogastric junction. Esophageal manometry measured a median lower esophageal sphincter resting tonus of 17.7 (5.9-28.5) mmHg (n =13). Sphincter relaxation in swallowing was absent in all patients. The contraction amplitude in the distal esophagus (mean value for 10 barium swallows) was 35.3 (6.0-65.3) mmHg at 5 cm above the lower esophageal sphincter, and 32.5 (10.0-122.8) mmHg at 10 cm.
The conventional histopathologic examination of the 15 biopsies from patients with achalasia did not demonstrate the presence of dysplasia. The immunohistochemical evaluation showed a median staining intensity with MIB-1 (basal) of 25% (0%-40%) (Figure 1), and was thus > 20% in 8 achalasia patients. Conversely, a positive reaction was noted in all patients with squamous cell carcinoma, at a median value of 70% (60%-80%). While all achalasia biopsies showed a negative reaction for p53, a positive reaction was observed for 60% of the carcinomas.
Figure 1 Immunohistochemical evaluation, staining with PAb 1801 for p53 and MIB-1 (basal) for Ki67 antigen in a patient with achalasia.
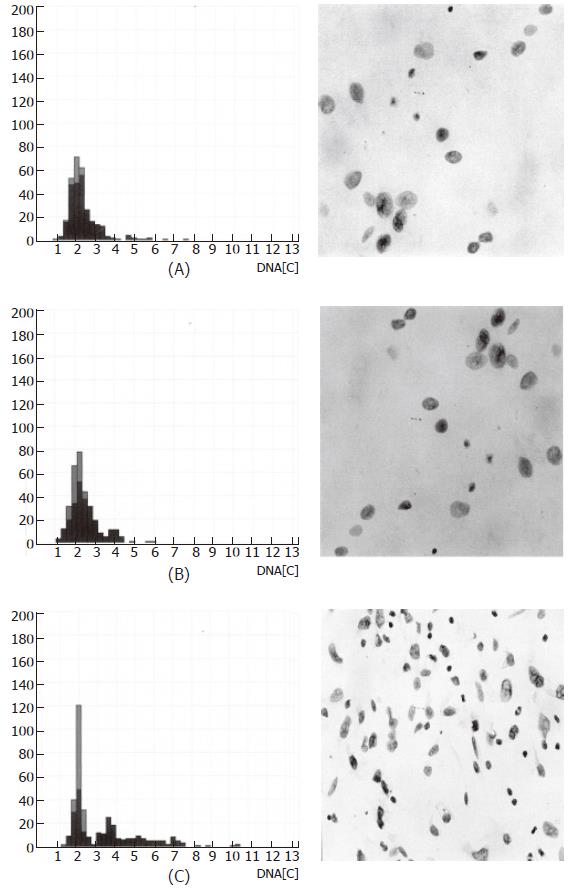
Image cytometric DNA analysis detected aneuploidy in 4 of 15 (26.7%) patients. In 9 of 15 (60%) of the peritumoral samples, aneuploidy was identified at 2 cm proximal to the tumor margin, and was present in all tumor specimens. The aneuploidy rate in achalasia was 0.9% (0%-9.4%) diploid and 0 (0%-2.4%) tetraploid. In the peritumoral tissue, the rate was 2.6% (0%-15.0%) diploid and 0 (0%-4.5%) tetraploid, compared to 12.5% (1.2%-48.0%) diploid and 1.4% (0%-15.0%) tetraploid at the center of the carcinoma. Grading of malignancy according to Boecking (scale of 0-3) was 0.2 (range 0.1-0.8) in patients with achalasia, 0.4 (range 0.1-1.5) in peritumoral tissue, and 1.3 (range 0.6-1.9) in the tumor center. The 5c-exceeding rate was 0.3% (0%-5%) in the achalasia specimens, 1.3% (0%-9.6%) in the peritumoral samples, and 10.5% (3.1%-32.6%) in the tumor center. Corresponding to the smaller sample numbers due to overlapping, the 7c- and 9c-exceeding rates were lower than the 5c-rate; there was a similar increase from achalasia to peritumoral samples and to carcinoma for these rates as that observed for the above factors.
Figure 2 shows the progression of DNA aneuploidy on the basis of nuclear morphology with the respective histogram for achalasia samples (A), in peritumoral tissue 2 cm proximal to the tumor border (B), and in the tumor center of the squamous cell carcinoma (C). The comparison of biopsies from patients with achalasia, and the peritumoral as well as the carcinoma specimens employing the Kruskal-Wallis test demonstrated statistically significant differences for the aneuploidy rate (diploid: P < 0.0001; tetraploid: P = 0.001), grading of malignancy according to Boecking (P < 0.0001), as well as the 5c- (P < 0.0001), 7c- (P < 0.0001), and 9c- (P < 0.0001) exceeding rates.
Figure 2 Image cytometric DNA analysis: histogram and nuclear morphology.
(A) achalasia; (B) peritumoral tissue (esophageal carcinoma); (C) tumor center (esophageal carcinoma).
There was no correlation in patients with achalasia between mucosal DNA alterations or proliferation markers and the presence of clinical symptoms, the Eckardt-Score, the maximum diameter of the esophageal body, or the resting pressure of the lower esophageal sphincter (P > 0.05). Following allocation of the patients with achalasia to 2 groups (patients with aneuploidy: n = 4 and without aneuploidy: n = 11), a difference in the diameter of the esophageal body becomes apparent, which is relevantly larger (42.5 vs 30 mm) in the patients with aneuploidy; however, no differences were observed with regard to persistence of symptoms, the Eckardt-Score, and resting pressure.
DISCUSSION
DNA aneuploidy and increased cell proliferation have an important role in the pathogenesis of esophageal carcinoma. The prognostic significance of aneuploidy has been described for squamous cell carcinoma and adenocarcinoma of the esophagus[20-22]. Even in the absence of positive findings on conventional histopathology, Image cytometry offers the detection of early mucosal DNA alterations and therefore serves as a useful tool in the selection of a defined group of patients at risk for malignancy. One of the advantages of image-directed cytometry over flow cytometry is that it enables measurement of the integrated optical staining density per specimen in a digitalized image, allowing the visual assessment of the individual measured objects with the exclusion of artifacts. The presence of aneuploidy in 27% of our patients with achalasia is in accordance with the increased malignancy rate known for this disorder, and provides the possibility of early tumor detection. In addition, the method used in this study, which is based on the modification of enzymatic nuclear isolation with pronase, represents a useful rapid diagnostic test for the identification of DNA alterations.
Conventional methods of nuclear isolation for Feulgen staining and subsequent image cytometric DNA diagnosis have primarily been performed in paraffin-embedded samples and were characterized by both a minimal yield of nuclei and low staining intensity for the small number of available nuclei[23,24]. The disadvantage of paraffin-embedded or formalin-fixed samples is that unavoidable artifacts and alterations in the cell structure occurring during nuclear isolation lead to falsification of the results[25,26]. The described modification of the pronase method for nuclear isolation from non-formalin-fixed native specimens used in this study was shown to be readily reproducible. This was due to the high number of cell nuclei, which were markedly in excess of the required number of 250 study and 30 reference nuclei, as well as to negligible clumping and clearly identifiable nuclear structures in the absence of artifacts. The evaluation of the samples prepared according to the described method in combination with additionally performed grading of malignancy proved to be highly reliable.
It has been speculated that precancerous alterations in achalasia develop as sequelae of a chronic inflammatory reaction of the esophageal squamous epithelium due to the retention and stasis of food and secretions. Conventional histomorphology has identified various mucosal alterations, including diffuse squamous epithelial hyperplasia, lymphocytic esophagitis, lymphocytic inflammatory alterations of the lamina propria mucosa, and the submucosa with prominent germinal centers, as well as periductal or glandular inflammatory reactions with complete loss of submucosal glandular structures[27,28]. The development of a multistep process from squamous epithelial hyperplasia to dysplasia, culminating in the manifestation of carcinoma may be hypothesized.
DNA aneuploidy in achalasia has previously been investigated only by one study, which observed a markedly lower number of chromosomal alterations (10%) than that found in the current investigation. As reported by Bektas et al[29], there were no pathologic findings in the control group of his study (0/18 controls). However, the method used by Bektas et al[29] was flow cytometry, whose limitations have been described elsewhere in this paper. The role of aneuploidy with regard to the neoplastic progression in achalasia-associated esophageal squamous cell carcinoma has been described in a case study carried out by Porschen et al[30] . In their patient, aneuploidy was also found in the mucosa adjacent to the carcinoma, a phenomenon which was likewise observed in the reference specimens from the carcinoma patients of our study. This finding may correspond to the model of epithelial lateral spread in squamous cell carcinoma leading to the development of multiple adjacent carcinomas of monoclonal origin described by Brieger et al[31]. Nevertheless, the time of occurrence, cellular interactions and induction by endogenous and exogenous noxae, and the distribution pattern of precancerous alterations in achalasia are currently not known.
Although dysplasia was not identified in the achalasia patients of this study - in correlation with DNA alterations - a positive reaction for Ki67 as a marker of increased proliferation was nevertheless found. Fujii et al[32] noted an increased number of Ki67-positive cells in the non-malignant epithelium of 4 patients with achalasia and esophageal carcinoma. While mutations of the p53 tumor suppressor gene are known to be of crucial importance for the pathogenesis of esophageal squamous cell carcinoma, the role of this gene in the development of achalasia-associated carcinoma is still under discussion. Conversely, immunohistochemical studies with p21, p16 and EGF (epidermal growth factor) have shown that dysplastic epithelium found in esophageal carcinoma represents a borderline alteration between hyperplasia and the carcinoma in situ in achalasia[33] .
DNA aneuploidy in patients with achalasia can be identified in 27% of cases in the absence of histomorpho-logically proven dysplasia using image cytometry. Marked overlapping of chromosomal alterations between achalasia biopsies with peritumoral tissue and the tumor center was found in individual patients with esophageal squamous cell carcinoma. The identification of DNA aneuploidy may thus be of major importance in the early diagnosis of carcinoma in achalasia. Further long-term studies in patients with aneuploidy are needed to answer the question as to the cost-benefit effectiveness of this procedure. Should the decision favor of the use of image cytometry as an early diagnostic tool be made at this early stage, the described method could serve as clinically relevant rapid diagnostic test.
S- Editor Pan BR L- Editor Kumar M E- Editor Ma WH