Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1774
Revised: October 28, 2005
Accepted: November 18, 2005
Published online: March 21, 2006
AIM: To investigate the effect of replication-incompetent adenovirus vector expressing MDA-7/IL-24 on tumor growth and apoptosis in human hepatocellular carcinoma (HCC) cell line HepG2 and normal liver cell line L02.
METHODS: We constructed the recombinant replication-incompetent Ad.mda-7 virus vector and infected it into the human HCC cell line HepG2 and normal liver cell line L02. RT-PCR was performed to detect the mRNA expressing in cells. by ELISA was used to detect MDA-7/IL-24 protein expression in the culture supernatant. The effect of apoptosis induced by Ad.mda-7 was confirmed by Hoechst staining and flow cytometry assay with Annexin-V and PI staining. MTT assay was used to determine growth inhibition of HepG2 cells, and cell-cycle and hypodiploidy analyses were performed by flow cytometry.
RESULTS: Recombinant replication-defective virus expressing MDA-7/IL-24 was constructed successfully. RT-PCR showed that the Ad.mda-7 could mediate the expression of the exogenous gene MDA-7/IL-24 into HepG2 and L02. The concentration of MDA-7/IL-24 protein in supernatant was 130 pg/mL and 110 pg/mL in Ad.mda-7-infected L02 and HepG2 cells, respectively. Ad.mda-7 infection obviously induced apoptosis (from 2.60±0.72% to 33.6±13.2%, P = 0.00012) and growth suppression in HepG2 (inhibition ratio IR = 68%) and an increase in the percentage of specific cancer cell types at the G2/M phase of the cell cycle (from 6.44% to 32.29%, P < 0.01), but not in L02 cells.
CONCLUSION: These results confirm selectively induction of apoptosis and growth suppression by the mda-7/IL-24 gene with replication-incompetent adenovirus vector in human hepatocellular carcinoma cell line HepG2.
- Citation: Wang CJ, Xue XB, Yi JL, Chen K, Zheng JW, Wang J, Zeng JP, Xu RH. Melanoma differentiation-associated gene-7, MDA-7/IL-24, selectively induces growth suppression, apoptosis in human hepatocellular carcinoma cell line HepG2 by replication-incompetent adenovirus vector. World J Gastroenterol 2006; 12(11): 1774-1779
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1774
Primary hepatocellular carcinoma (HCC) is one of the most common lethal malignant tumors in the world[1], causing an estimated 1 250 000 deaths every year worldwide. Unfortunately, about 50% new cases are from China. The clinical therapies for HCC include surgical resection and liver transplantation, but only few HCC patients can receive these treatments. Moreover, the recurrent rate is very high even the patients received surgical treatments. Besides clinical therapies, the gene therapies for HCC are running, including transgenic therapy by antioncogenes such as p53 and Rb, anti-sense nucleotide technique, drug gene therapy such as suicide gene-like HSV-TK, tumor vaccine, and so on. However, the clinical effect of gene therapies was limited because these genes were not specific to tumor cells, which means they kill the normal cells and the tumor cells simultaneously. The research on the treatment protocol selectively killing tumor cells but not influencing normal cells has become a hot topic of research on tumor treatment[2]. Melanoma differentiation-associated gene-7 (MDA-7)/IL-24 was identified by a combination of recombinant fibroblast interferon (IFN-b) and the protein kinase C activator mezerein (MEZ) subtraction hybridization by Fisher in 1995[3], according to the chromosomal location on 1q32, the presence of a secretory signal, its association with specific cells of the immune system, and the ability of MDA-7 protein to act as an immune modulator. Now mda-7 has been renamed IL-24[4-7].
Some studies indicated that over-expression of mda-7/IL-24 by a replication-defective adenovirus vector results in growth suppression and apoptosis in a broad range of different carcinoma cell lines, including mesotheliomas[8], osteosarcoma[8], melanoma[2,3,9,10], and carcinomas of the lung[11,12], breast[13], pancreas[14], glioblastoma[15,16] and prostate[17]. The anti-tumor effects were independent of the genomic status of p53, RB, p16[2]. Although MDA-7/IL-24 has the properties potentiality kill many different types of cancer cells, it has not any harmful effects in many kinds of normal cells. These unique potentiality of mda-7/IL-24 suggested that this gene could prove beneficial for cancer gene therapy[2]. In the present study, we investigated the impact of Ad.mda-7 on growth, cell cycle and survival of human HCC cell line HepG2 and normal liver cell line L02, resulting in selectively induction of apoptosis and growth suppression by the mda-7/IL-24 gene with replication-incompetent adenovirus vector. In these contexts, the study provides important support to the use of Ad.mda-7 for selective cancer gene therapy for HCC.
Human HCC cell line HepG2 and normal human liver cells line L02 (gift from Dr Guanjian) were cultured in high glucose DMEM supplemented with 100 mL/L fetal bovine serum (FBS) at 37°C in a humidified incubator containing 50 mL/L CO2 95% in air.
The recombinant replication-defective Ad.mda-7 virus was created in our laboratory. Briefly, human MDA-7/IL-24 cDNA was directionally cloned into pSGCV to produce pSGCMV-MDA7. By using plasmid transfection method, the pSGCMV-MDA7 and adenovirus skeletal plasmid were co-transfected to HEK293 cells to construct the recombinant adenovirus vector Ad.mda-7, carrying MDA-7/IL-24 gene, by intracellular homologous recombinant. The genomes were analyzed to confirm the recombinant structure and then the virus was plaque purified and amplificated in 293 cells.
After cells infected with 1 000 VP/cell (virus particle/cell) of Ad.vec and Ad.mda-7, respectively, were harvested at 48 h, total RNA was extracted from cells using the Qiagen RNeasy mini kit (USA) according to the manufacturer’s protocol. Primers used in PCR were designed according to the reported IL-24 cDNA sequence. The primer sequences were 5’-GGGCTGTGAAAGACACTAT-3’ (forward) and 5’-GCATCCAGGTCAGAAGAA-3’ (reverse). The primer sequences of β-actin were 5’-CCTTCCTGGGCAATGGAGTCCT-3’ (forward) and 5’-GGAACAATGATCTTGATCTT-3’ (reverse). The reaction mixture with corresponding primers was amplified through 30 cycles, each cycle consisting of denaturation at 94°C for 30 s, primer annealing at 55°C for 30 s, and extension at 72°C for 30 s. Cycles were preceded by incubation at 95°C for 3 min to ensure the full denaturation of the target gene and also an extra incubation at 72°C for 5 min to ensure full extension of the product. The products of PCR were analyzed on 10 g/L agarose gel electrophoresis.
The cell culture supernatant was collected and stored at -20°C until use. A total of 100 µL of the supernatant was added to Microplate wells, and 200 µL of anti IL-24 (Biotin USA) was added 10 s later. After incubation at 37°C for 30 min, the sample was washed 5 times with wash buffer, followed by addition of 200 µL of HRP to each well following an incubation at 37°C for 30 min. Then the wells were washed 5 times, followed by addition of 100 µL of TMB and incubation in room temperature with protection from light. Twenty minutes later, 100 µL of stop solution was added, and the absorbance was read on a microplate reader at 450 nm. All experiments were performed in duplicate. Finally, we calculated the value of results with standard curve.
Cells were seeded in 96-well tissue culture plates (1x103 cells/well) and treated with PBS, 1 000 VP/cell of Ad.mda-7 and Ad.vec (1 000 VP/cell), respectively, at the next day. At the indicated time points, the medium was removed, and fresh medium containing 0.5 mg/mL MTT (Roche Diagnostics GmbH Co., Germany) was added to each well. The cells were incubated at 37°C for 4 h, followed by addition of about 150 µL of solubilization solution (0.01 mol/L HCl in 100 g/L SDS) to each well, and incubation of cells for a further 10 min at 37°C with gentle shaking. The optical density of the plates was read on a microplate reader at 540 nm.
After 48 h of infection, cells were washed once with PBS and fixed in 40 g/L paraformaldehyde for 30 min at room temperature. After two washes with PBS, cells were stained for 30 min in the dark at room temperature with 0.05 mg/mL Hoechst 33258 (Sigma USA) in PBS. Nuclear fragmentation was visualized using a fluorescence microscope equipped with a UV-2A filter and Olympus BX60 photographic camera. Apoptotic cells were identified by condensation of nuclear chromatin and its fragmentation.
Cells were trypsinized and washed once with complete media. Aliquots of cells (5×105) were resuspended in complete media (0.5 mL) and stained with FITC-labeled Annexin-V (Jinmei Co., China) according to the manufacturer’s instructions. Propidium iodide (PI) was added to the samples after staining with Annexin-V to distinguish late apoptotic and necrotic cells. Flow cytometry (Becton Dickinson, San Jose, CA, USA) was performed immediately after staining.
Cells were cultured as aforementioned. After reaching 30% confluence, the cells were treated with DMEM without FCS for 24 h for synchronization. At the next day, cells were treated with PBS, Ad.vec and Ad.mda-7, respectively. Forty-eight hours later, cells were trypsinized, washed with PBS and fixed in 700 mL/L ethanol overnight at -20°C. Cells were then washed with PBS, and aliquots of 1x106 cells were resuspended in 1 mL of PBS containing 1 mg/mL of RNase A and 0.5 mg/mL of PI. After 30 min of incubation, cells were analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA).
All the experiments were performed at least three times. The results were expressed as mean ± SE. Statistical comparisons were made using an unpaired two-tailed Student’s t test. A P < 0.05 was considered statistically significant.
To determine the efficiency of transgenic expression, HepG2 and L02 cells were infected with Ad.vec and Ad.mda-7, and mRNA expression was detected using RT-PCR (Figure 1). The results suggested that the expression of MDA-7/IL-24 mRNA could be detected in both HepG2 and L02 cell lines infected by Ad.mda-7 but not by Ad.vec or control team. MDA-7/IL-24 gene could be expressed in cells with infected with Ad.mda-7.
Secreting MDA-7/IL-24 protein was confirmed by ELISA assay after Ad.mda-7 infection. After 48 h of L02 and HepG2 cells infection with Ad.mda-7, the concentration of MDA-7/IL-24 protein in the supernatant was detected 130 pg/mL and 110 pg/mL, respectively, whereas no MDA-7/IL-24 protein expression was detected in L02 and HepG2 cells treated with PBS and Ad.vec.
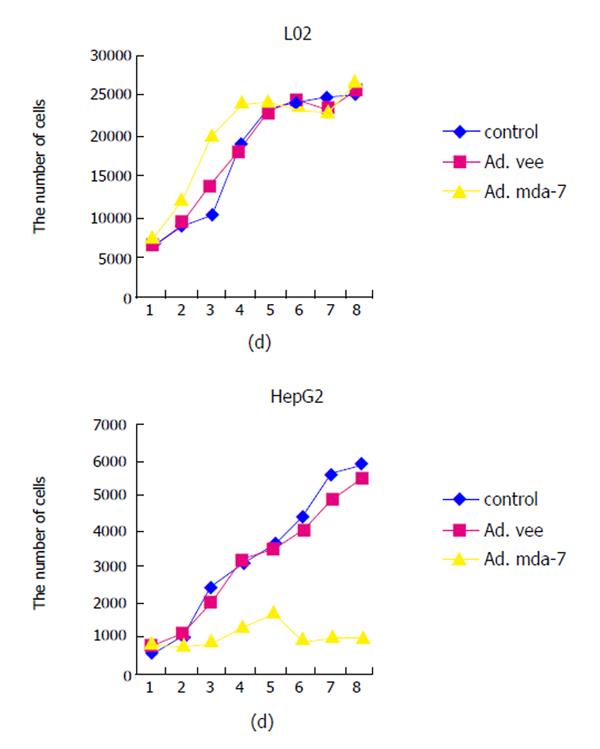
HCC cell line HepG2 and normal liver cell line L02 were infected with Ad.mda-7 and proliferation and cell viability were determined by MTT. As shown in Figure 2, no proliferation arrest effect was observed on normal liver cell line L02 of Ad.vec, Ad.mda-7 or control groups. However, the anti-proliferative activity of Ad.mda-7 was readily apparent in HCC cell line HepG2, and the inhibition ratio was 68%.
We observed that Ad.mda-7 infection induced apoptosis of human HCC cell line HepG2. As shown in Figure 3, a significantly higher apoptotic rate was observed in HepG2 cells infected with Ad.mda-7 (48%) compared to those infected with Ad.vec (1.6%) and the control group (1.1%) (counted 1 000 cells consecutively) (P < 0.005), whereas no apparent changes were observed in normal liver cell L02, the rate of apoptosis being 1.7%, 1.9% and 2.1%, respectively (P > 0.05). These data indicated that MDA-7/IL-24 could induce apoptosis in HepG2.
Annexin-V and PI staining assays with flow cytometry quantified the effect of Ad.mda-7 on apoptosis induction in human HCC cell HepG2 and normal liver cell L02 (Table 1). We observed a significantly increased percentage of apoptotic HCC cells HepG2 infected with Ad.mda-7 as compared to HepG2 infected with Ad.vec and the control cells. In contrast, L02 cells did not show significantly increased apoptotic rate after being infected with Ad.mda-7. Thus, these results suggested that Ad.mda-7 infection could kill HCC cells but not normal liver cells.
| L02 | HepG2 | |||||
| Control | Ad.vec | Ad.mda-7 | Control | Ad.vec | Ad.mda-7 | |
| Early apoptotic cells | 110 ± 23 | 120 ± 36 | 100 ± 11 | 50 ± 37b | 300 ± 85d | 1 990 ± 430 |
| Late apoptotic cells | 60 ± 14 | 100 ± 21 | 130 ± 15 | 10 ± 40b | 200 ± 63d | 1 370 ± 902 |
| Total cells | 170 ± 28 | 220 ± 45 | 230 ± 16 | 60 ± 72b | 500 ± 150d | 3 360 ± 132 |
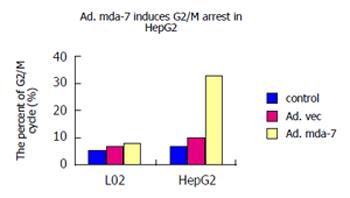
Cell cycle phase assayed by flow cytometry after the fixed cells were stained with PI. As shown in Figure 4, Ad.mda-7 markedly induced a G2/M accumulation in HepG2 cell lines, showing that the rates of G2/M phase cells were 32.29% in Ad.mda-7 group, 10.30% in Ad.vec and 6.44% in control groups (P < 0.01). However, the rates of G2/M phase cells were 7.95%, 6.65% and 5.54% in L02 cells of Ad.mda-7, Ad.vec and control groups, respectively (P > 0.05), demonstrating only minimal G2/M accumulation in normal liver cells L02. Thus, these results suggested that Ad.mda-7 infection could significantly induce an increase in the percentage of HCC cell line HepG2 in the G2/M phase of the cell cycle, but not in normal liver cell line L02.
A study by Jiang et al[3] demonstrated that melanoma differentiation-associated gene-7 (MDA-7/IL-24) was highly expressed in melanocytes, but low expressed in melanoma cells, and because of the inducing capacity of growth arrest and differentiation in human melanoma cell line, named MDA-7. After the MDA-7/IL-24 was transfected into malignant melanoma cells, it could obviously block the growth, and promote apoptosis and differentiation, even reverse the malignancy of the melanoma[3]. Now it is clear that the MDA-7/IL-24 gene has been localized to chromosome 1q32 and its exon/intron structure has been established. Mda-7 is composed of seven exons with the first exon being noncoding. The chromosomal assignment and gene structure have been independently confirmed[4-6]. The region includes IL-10, IL-19, IL-20 and mda-7[4]. Moreover, based on structure of protein and the receptor, the gene has been reclassified and grouped into a newly recognized family of IL-10-related interleukins, IL-24[7]. Several independent studies have demonstrated that over-expression of the MDA-7/IL-24 gene, using vectors either plasmid or a replication-defective adenovirus, resulted in growth arrest and induction of apoptosis in a broad range of cancer cells, including lung cancer[11,12], breast cancer[13], pancreatic cancer[14], glioma[15,16], and prostate cancer[17]. Some signal transduction pathway and molecules have been reported as being regulated during mda-7-induced tumor suppression, including activation of the caspase cascade, PKR, p38, STAT3, PI3K, GSK-3, ILK-1, BAX, BAK, Fas, DR4, TRAIL, inducible nitric oxide synthase (iNOS), IRF-1, IRF-2 and p53[8]. Treatment of tumor cells with Ad-mda7 resulted in an increase in cells in the G2/M cell cycle phase[2]. In comparison to the antioncogenes, such as p53, the inhibitory effect of MDA-7/IL-24 on the growth of cancer cells was not related with the state of antioncogenes in these cancer cells (p53, Rb, or p16ink4)[2,11,14], so it could be used in cancer treatment more effectively without the influence in the expression of these anticancer genes in various kinds of cell lines. Surprisingly, the previous studies revealed that MDA-7/IL-24 gene had no any toxic and side effects on the normal cells, such as epidermal cells, lung fibroblasts, breast cells, prostate and lung epithelia, astrocytes, endothelia, melanocytes and so on[2,3], suggesting its selective property to malignant tumors. Therefore, MDA-7/IL-24 is considered a unique cytokine–tumor suppressor in the IL-10 family and there is no any toxic effect in normal cells, suggesting it as a perfect gene for use as a human cancer gene therapy.
In this study, the replication-incompetent adenovirus vector carrying MDA-7/IL-24 was successfully constructed and transfected into human normal liver cell line L02 and HCC cell line HepG2. Its effects on the two kinds of cells were observed, which provided the theoretical foundation for its application in the gene therapy for HCC in clinical practice. RT-PCR indicated MDA-7/IL-24 was successfully transfected into L02 and HepG2 cells with Ad.mda-7, but the control and Ad.vec groups could not show the mRNA expression. The protein expression was confirmed by ELISA assay and the effect was very different, although protein expression was seen both in L02 and HepG2. MTT assay revealed the capability of Ad.mda-7 in tumor growth arrest of HCC cell line HepG2, but not of normal liver cell line L02, indicating that MDA-7/IL-24 induces growth arrest only in HCC cells. Like the pervious studies, Ad.mda-7 induced a G2/M accumulation in HCC cell line HepG2, but not in normal liver cell line L02. Moreover, infection with Ad.mda-7 could increase the percentage of apoptotic cells apparently in HCC cells. On contrary, no increased percentage of apoptotic cells appeared in L02 cells.
In conclusion, Ad.mda-7 can induce the gene MDA-7/IL-24 expression in normal liver cells and hepatocellular carcinoma cells. Over-expression of MDA-7/IL-24 obviously induces the apoptosis and growth suppression in hepatocellular carcinoma cell line HepG2, without any toxic effect on normal liver cell line L02. These findings provide support for future clinical applications of MDA-7/IL-24 in the gene therapy of hepatocellular carcinoma.
The authors thank professor Fisher (Michael and Stella Chernow, Urological Cancer Research Scientist in the Departments of Pathology, Neurosurgery and Urology, Columbia University, USA) for his instruction, and prof. Jian-Hua Zhou (Laboratory of Nephropathy, Department of Pediatrics, Tongji Hospital) for his technical instructions and assistance.
S- Editor Wang J L- Editor Kumar M E- Editor Ma WH
| 1. | Venook AP. Treatment of hepatocellular carcinoma: too many options. J Clin Oncol. 1994;12:1323-1334. [PubMed] [Cited in This Article: ] |
| 2. | Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P. mda-7/IL-24: exploiting cancer's Achilles' heel. Mol Ther. 2005;11:4-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477-2486. [PubMed] [Cited in This Article: ] |
| 4. | Chang C, Magracheva E, Kozlov S, Fong S, Tobin G, Kotenko S, Wlodawer A, Zdanov A. Crystal structure of interleukin-19 defines a new subfamily of helical cytokines. J Biol Chem. 2003;278:3308-3313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Dumoutier L, Renauld JC. Viral and cellular interleukin-10 (IL-10)-related cytokines: from structures to functions. Eur Cytokine Netw. 2002;13:5-15. [PubMed] [Cited in This Article: ] |
| 6. | Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol. 2004;4:577-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ, Alexandre D. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051-7063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Gopalkrishnan RV, Sauane M, Fisher PB. Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. Int Immunopharmacol. 2004;4:635-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Allen M, Pratscher B, Roka F, Krepler C, Wacheck V, Schöfer C, Pehamberger H, Müller M, Lucas T. Loss of novel mda-7 splice variant (mda-7s) expression is associated with metastatic melanoma. J Invest Dermatol. 2004;123:583-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A. 2002;99:10054-10059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Kawabe S, Nishikawa T, Munshi A, Roth JA, Chada S, Meyn RE. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53-independent mechanisms. Mol Ther. 2002;6:637-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK. Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells. Surgery. 2004;136:437-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, Dent P, Fisher PB. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene. 2005;24:585-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P, Fisher PB. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Yacoub A, Mitchell C, Hong Y, Gopalkrishnan RV, Su ZZ, Gupta P, Sauane M, Lebedeva IV, Curiel DT, Mahasreshti PJ. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, Reed JC, Fisher PB. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138-8144. [PubMed] [Cited in This Article: ] |