Published online Feb 28, 2005. doi: 10.3748/wjg.v11.i8.1115
Revised: July 20, 2004
Accepted: September 19, 2004
Published online: February 28, 2005
AIM: To assess the effect of non-selective ETA/B (LU 302872) and selective ETA (LU 302146) antagonist on pancreatic histology and ultrastructure of acinar cells in connection with trypsinogen activation in early caerulein-induced AP.
METHODS: Male Wistar rats with caerulein-induced AP, lasting 4 h, were treated i.p. with 10 and 20 mg/kg b.w. of each antagonist. Edema, inflammatory infiltration, necrosis and vacuolization of acinar cells in the pancreas were scored at 0-3 scale. Free active trypsin (FAT), total potential trypsin (TPT) after activation with enterokinase, and index of trypsinogen activation (%FAT/TPT) were assayed in pancreatic homogenates.
RESULTS: In untreated AP, the edema, inflammatory infiltration, necrosis and vacuolization increased as compared to control healthy rats (P<0.01). None of the treatment exerted any meaningful effect on the edema and inflammatory infiltration. The selective antagonist increased slightly the necrosis score to 0.82±0.06 at higher dose (P<0.05) vs 0.58±0.06 in untreated AP. The non-selective antagonist increased slightly the vacuolization score to 2.41±0.07 at higher dose (P<0.01) vs 1.88±0.08 in untreated AP. The decrease in the number of zymogen granules, disorganization of endoplasmic reticulum, autophagosomes and cytoplasmic vacuoles were more prominent in treated AP than in untreated AP groups. %FAT/TPT in untreated AP increased about four times (18.4±3.8 vs 4.8±1.3 in control group without AP, P<0.001). Treatment of AP with both antagonists did not affect significantly augmented trypsinogen activation.
CONCLUSION: The treatment with endothelin-1 receptors (non-selective ETA/B and selective ETA) antagonists has essential effect neither on the edema and inflammatory infiltration nor on trypsinogen activation observed in the early course of caerulein-induced AP. Nevertheless a slight increase of the necrosis and vacuolization score and some of the ultrastructural data could suggest the possibility of their undesired effects in caerulein-induced AP at investigated doses.
- Citation: Andrzejewska A, Dlugosz JW, Augustynowicz A. Effect of endothelin-1 receptor antagonists on histological and ultrastructural changes in the pancreas and trypsinogen activation in the early course of caerulein-induced acute pancreatitis in rats. World J Gastroenterol 2005; 11(8): 1115-1121
- URL: https://www.wjgnet.com/1007-9327/full/v11/i8/1115.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i8.1115
The role of endothelin-1 (ET-1), as a potent vasoconstrictor polypeptide, and its receptor antagonists in acute pancreatitis (AP) has been an intensively studied issue recently. The experimental studies have brought varied results and evoked a number of controversies. In many experiments, the application of selective and non-selective ET-1 receptor A and B antagonists caused a significant improvement in pancreatic capillary blood flow, a decrease in capillary permeability and a reduction in leukocyte rolling in mild and severe AP[1-5]. A beneficial effect of the ET-1 receptor antagonists was also evidenced by less pronounced morphological changes in AP, including necrosis and vacuolization of acinar cells, hemorrhagic changes and granulocytic infiltration of pancreatic parenchyma[3,6-9]. Foitzik et al[10] found a considerable drop in the mortality rate of rats after the application of the selective ET-1 receptor A antagonist, LU 135252, in severe AP.
However, not all researchers have confirmed the beneficial effects of ET-1 receptor antagonists in AP. The results of Martignoni et al[11] clearly indicated that selective ET-1 receptor A (ETA) antagonist (LU 135252) did not decrease but showed a tendency to increase mortality in animals with taurocholate-induced pancreatitis. A study by Fiedler et al[12] revealed no effect of non-selective ET-1 receptor A and B (ETA/B) antagonist, bosentan, on the survival rate of rats or on the morphological and biochemical changes in severe taurocholate AP. Todd et al[9] observed no decrease in the mortality rate of animals in acute hemorrhagic pancreatitis after the application of non-selective ET-1 receptor A and B antagonist PD 145065, despite decreased severity of inflammation. Also recent studies have not confirmed the advantageous effect of non-selective (ETA/B) and selective (ETA) blockade in necrotic or edematous AP[11].
On the other hand, Kogire et al[13] found a significant improvement in the course of caerulein-induced pancreatitis following systemic infusion of ET-1 by evaluating serum α-amylase level, dry/wet weight ratio, and histological changes. In contrast, endothelin receptor blockade with BQ 123, a potent selective ETA receptor antagonist, augmented pancreatic edema and the extent of inflammatory cell infiltration. The above data indicate that the role of ET-1 and ET-1 receptors antagonists in AP remains controversial and not fully elucidated.
Therefore, the aim of the present study was to evaluate and compare the effect of non-selective ET-1 A and B receptors (ETA/B) antagonist, LU 302872, and selective receptor A (ETA) antagonist, LU 302146, on the morphological and ultrastructural changes and trypsinogen activation in the pancreas in early caerulein-induced edematous, AP in rats.
The experiments were carried out on 40 male Wistar rats, 240-280 g of body weight, housed individually in wire bottomed cages, at a room temperature of 21±1 °C using a 12-h light-dark cycle. They were fed with a laboratory chow diet and fasted overnight, before the experiment, with free access to water. Care was provided in accordance with the current procedures for the care and use of laboratory animals. The protocol has been approved by the local Bioethical Commission.
Acute pancreatitis was induced according to the method of Yamaguchi et al[14]. The rats were injected with caerulein (Sigma Chemical Co., St. Louis, MO, USA) at a dose of 40 μg/kg of body weight intraperitoneally (i.p.) twice, at 1 h interval. In control rats, only vehiculum of caerulein (saline solution) was given i.p. In the treated rats, the solution of respective ET-1 receptor antagonist in vehiculum was given i.p. simultaneously with the first injection of caerulein.
Rats were subdivided into six groups as follows:
Group 1. Control group (C), received only saline solution i.p. at 0 and 1 h (n = 6).
Group 2. Rats with untreated caerulein-induced AP, received only saline solution i.p. as in the control group (n = 10).
Group 3. Rats with caerulein-induced AP treated with the non-selective antagonist of ET-1 A and B receptors: LU 302872 (ETA/B antagonist) at a dose of 10 mg/kg b.w. i.p. given at the beginning of the experiment , at the same time as the first injection of caerulein (n = 6).
Group 4. Rats with caerulein-induced AP treated with LU 302872 at a dose of 20 mg/kg b.w., i.p. given at the beginning of the experiment, at the same time as the first injection of caerulein (n = 6).
Group 5. Rats with caerulein-induced AP treated with selective ET-1 A receptor antagonist LU 302146 (ETA antagonist), at a dose of 10 mg/kg b.w. i.p., at the beginning of the experiment, at the same time as the first injection of caerulein (n = 6).
Group 6. Rats with caerulein-induced AP treated with LU 302146, at a dose of 20 mg/kg b.w. i.p., at the beginning of the experiment, at the same time as the first injection of caerulein (n = 6).
The volume of saline as a vehiculum was equilibrated in all groups to 2×2 mL/kg b.w. The ET-1 receptor antagonists were generously donated by Knoll AG, Ludwigshafen, Germany (Dr. M. Kirchengast).
The animals were killed by quick decapitation after 4 h after the first injection of caerulein, in general anesthesia induced with i.p. ketamine 40 mg/kg b.w. and pentobarbital 20 mg/kg b.w. The pancreases were quickly excised, freed from the peripancreatic tissues and weighed. The specimens for histological and ultrastructural examinations were taken. The remaining portion of the pancreas was processed for biochemical assays.
The representative specimen of the pancreatic tissue from each rat was fixed in 10% neutral-buffered formalin. Sections of the samples were stained with H&E and examined under light microscope at ×200 magnification - intermediate power field (IPF) by a blinded observer in a hundred fields from each group. Histological changes were evaluated according to Kyogoku et al[15]. Interstitial edema was scored as follows: 0 = absent, 1 = expanded interlobular septa, 2 = expanded intralobular septa, 3 = separated individual acini. Polymorphonuclear neutrophils (PMNs) infiltration was scored as 0 = absent, 1 = less than 20 PMNs per IPF, 2 = 20-50 PMNs per IPF and 3 = more than 50 PMNs per IPF. Parenchymal necrosis was scored as the percentage involvement of the examined area: 0 = absent, 1 = less than 5% IPF, 2 = 5-20% IPF, 3 = more than 20% IPF. The grading of vacuolization was based on the percentage of acinar cells with cytoplasmic vacuoles per IPF: 0 = absent, 1 = less than 20%, 2 = 20-50%, 3 = more than 50%. Hemorrhagic changes were absent and therefore not scored.
Small specimens (about 1 mm3) of pancreatic tissue (three from each animal) were immediately fixed in 3.6% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) for 3 h and after washing in a buffer, postfixed in 2% osmium tetroxide for 1 h. The samples were dehydrated in alcohol and propylene oxide and then embedded in Epon 812. The ultrathin sections were cut from each block on a Reichert ultramicrotome, stained with lead citrate and uranyl acetate, and studied under an Opton 900 PC transmission electron microscope field by field. Fifty to 60 electron micrographs of the most characteristic changes from each group were made. The determination of pathology was made blind.
The remaining pancreatic tissue was homogenized in ice-cold four volumes of 50 mmol/L Tris-HCl buffer (pH 8.0), containing nonorganic detergent Triton X-100, 5 mL/L during 1 min by three full up and down strokes using a motor-driven glass-Teflon homogenizer (Thomas Scientific, Swedesboro, NJ, USA) cooled with ice. The resulting homogenate was sonified for 20 s in an ice bath using Vibra cell, model VC 50, Sonics and Materials Inc., Danbury, CT (frequency 20 kHz and amplitude 70). The volumes were then adjusted giving 10% homogenates placed on ice.
Free active trypsin (FAT) and total potential trypsin (TPT) in whole homogenate were assayed according to Yamaguchi et al[14] with the exception that Nα-p-tosyl-L-arginine methyl ester hydrochloride (TAME) 1 mmol/L was used as a substrate and the absorbance of released product was estimated at 247 nm wave length in an automatic spectrophotometer Pye Unicam SP 505 (Cambridge, UK), as in our previous studies[16, 17].
TPT in whole homogenate was estimated after the activation of trypsinogen with enterokinase in 1:1 dilution in 50 mmoL Tris-HCl buffer, pH 8.0 for 30 min at 37 °C. The freshly prepared working solution of enterokinase contained 2 mg of enzyme/mL of the same buffer. The time of activation proved to be sufficient for complete activation. The activity was expressed in μg of trypsin/mg of protein by comparison with the calibration curve of increasing concentrations of bovine trypsin, type I. The %FAT/TPT ratio served as an index of trypsinogen activation[14].
All reagents for biochemical assays were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Histologic data were expressed as the range of the scores and mean±SE and compared using non-parametric tests: the Mann-Whitney for two groups and the Kruskal-Wallis for multiple groups. The results of biochemical assays were reported as mean±SE and after performing an F test for the equality of variances, the means were compared using the t test for unpaired data. The differences with P<0.05 were considered statistically significant. SPSS 8.OPL statistical program was used (SPSS Inc., Chicago, IL).
The microscopic picture of the pancreas in the control group (C) showed only negligible swelling of pancreatic interstitial tissue with the presence of single neutrophils. Rats with untreated AP displayed moderate edema and inflammatory infiltration of neutrophils. Moreover, a necrosis of some of the acinar cells and their marked vacuolization were found. The changes in all AP animals were significantly more pronounced (P<0.001) than in healthy animals. Assuming the increase of score from 0 to 3 points as 100%, the treatment of AP with ET-1 receptor antagonists did not reduce the pancreatic edema and even a slight increase (<10%) of its score after higher dose of non-selective ETA/B receptor antagonist was noted. Only slight reduction of PMNs infiltration (<10%) after higher dose of the selective antagonist compared to untreated AP or treated with the non-selective antagonist was noted. The necrosis of acinar cells was slightly more pronounced (6-13%) after the application of both doses of the selective receptor A antagonist than in the untreated group or in the groups treated with both doses of non-selective ETA/B antagonist. Mean vacuolization score was slightly increased after both doses of non-selective (12-18%) and after higher dose of selective ET-1 receptors antagonists (10%) as compared to untreated AP. The mean scores of histological changes are reported in Table 1.
| No. | Group | Edema | PMNs infiltration | Necrosis | Vacuolization |
| 1 | Control (C) (n = 6) | (0-1) 0.12±0.03 | (0-1) 0.06±0.02 | (0) 0.00±0.00 | (0-1) 0.08±0.03 |
| 2 | Untreated AP (n = 10) | (1-3) 2.04±0.07b | (03) 1.61±0.08b | (0-2) 0.58±0.06b | (1-3) 1.88±0.08b |
| 3 | AP + LU 302872 10 mg/kg (n = 6) | (1-3) 2.02±0.07b | (1-3) 1.66±0.07b | (0-1) 0.48±0.05b | (1-3) 2.24±0.08bd |
| 4 | AP + LU 302872 20 mg/kg (n = 6) | (1-3) 2.30±0.06bdh | (0-3) 1.68±0.08b | (0-2) 0.50±0.05b | (1-3)2.41±0.07bd |
| 5 | AP + LU 302146 10 mg/kg (n = 6) | (0-3) 1.96±0.09b | (0-3) 1.43±0.09be | (0-2) 0.86±0.07bdf | (1-3)1.91±0.09bf |
| 6 | AP + LU 302146 20 mg/kg (n = 6) | (0-3) 2.14±0.07b | (0-3)1.41±0.07bce | (0-2) 0.82±0.06bdf | (1-3)2.19±0.07bdeg |
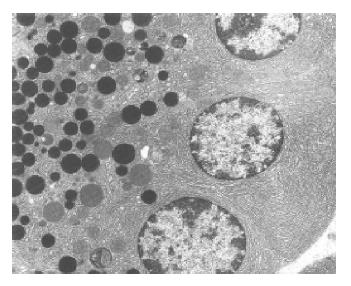
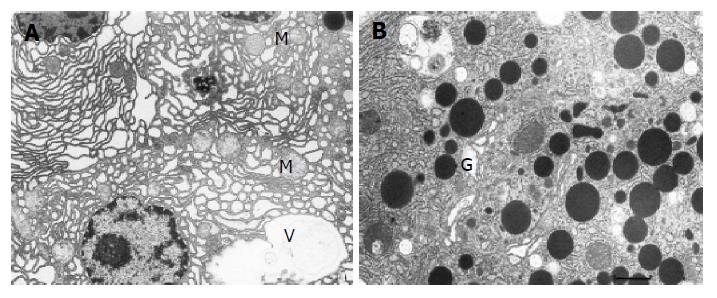
Group 1 (control group) The ultrastructural picture of acinar cells did not show any deviations from the norm (Figure 1). The interstitial tissue displayed slight swelling and sporadic neutrophils and erythrocytes. Blood vessels were normal in appearance.
Group 2 (untreated AP) Acinar cells of the pancreas displayed features of damage of varied intensity. Many of them had dilated, disorganized, and partially degranulated rough endoplasmic reticulum (RER) channels (Figure 2A). The cisternae of the Golgi apparatus were sometimes distended and filled with flocculent material. Zymogen granules were rare, varying in size and electron density (Figure 2B) and frequently migrated to the basolateral parts of the cell. Some of the mitochondria showed increased matrix translucence and destruction of their cristae. The majority of pancreatic acinar cells contained vacuoles, which sometimes reached large sizes and occupied a considerable area of the cell. Moreover, the cytoplasm contained autophagosomes with fragments of cellular organelles or sometimes zymogen granules. A necrosis of acinar cells was only sporadically observed.
The interstitial tissue showed marked edema and quite numerous inflammatory cells. Some of endothelial cells were swollen, with features of destruction of their organelles.
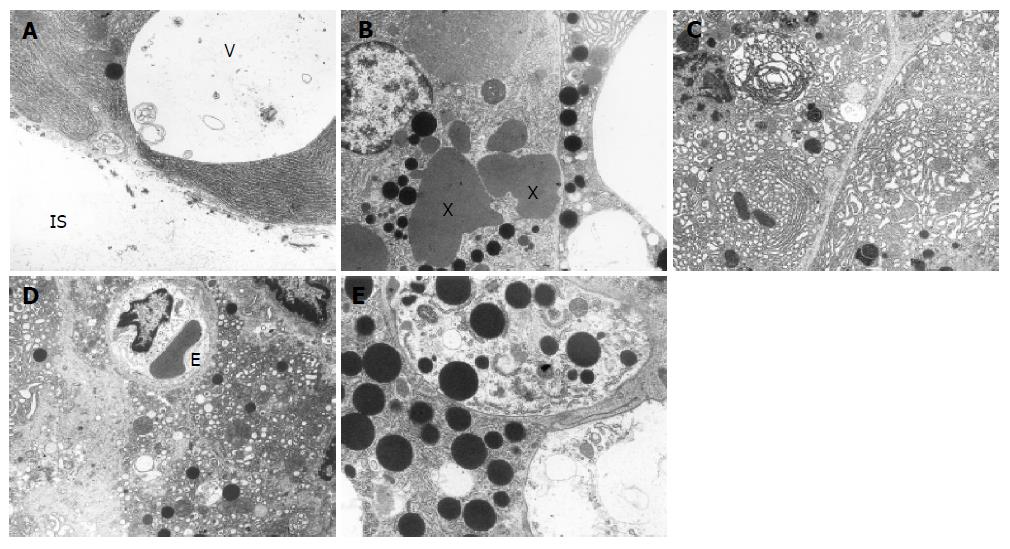
Groups 3 and 4 (AP treated with ETA/B antagonist) In both groups treated with non-selective ETA/B antagonist at a dose of 10 and 20 mg/kg, pancreatic acinar cells displayed slightly higher degree of damage than in the untreated AP. The cytoplasm contained quite numerous vacuoles some of them migrated to the base of the cells as if opening to the interstitial space (Figure 3A). These vacuoles were usually empty or contained membranous structures. Moreover, typical autophagous vacuoles were quite common and they contained RER fragments, damaged mitochondria, sometimes zymogen granules and amorphous or membranous structures. Zymogen granules were rare, varying in size and electron density, sometimes fused together to form irregular “lakes” (Figure 3B). Channels of RER were usually dilated and showed vesicular transformation, concentric arrangement, partial degranulation and focal total disorganization (Figure 3C). The cisternae of the Golgi apparatus were frequently dilated. Some mitochondria showed features of swelling. Necrosis of acinar cells was sporadic.
The interstitial tissue displayed distinct swelling, fibrin strands, inflammatory cells and features of damage in some endothelial cells. Generally the ultrastructural changes were similar after both doses of ETA/B antagonist and they were a bit more prominent than in the untreated group with AP.
Group 5 and 6 (AP treated with ETA antagonist).
Ultrastructural changes after treatment with lower dose of selective ETA antagonist resembled those observed in animals treated with both doses of non-selective ETA/B antagonist.
However, after treatment with higher dose, the ultrastructural alterations were more pronounced. Many acinar cells showed features of total disintegration and their fragments could be seen in the interstitial space (Figure 3D). The cytoplasm contained numerous vacuoles varying in size. Zymogen granules had varied electron density, frequently migrated to the base of the cell, and sporadically merged to form large irregular “lakes”. Sometimes they were found in autophagous vacuoles (Figure 3E) or were lying loosely in the interstitial space. Changes in RER, Golgi apparatus, mitochondria and in the interstitial tissue were similar to those noted in the previous groups.
In pancreatic homogenate (Table 2) in untreated AP this index was over four times as high as in control group (P<0.01). Treatment with non-selective ETA/B antagonist and selective ETA antagonist at both doses did not affect significantly the augmented trypsinogen activation index, compared to the untreated AP group.
| Group | FAT(mg/mg protein) | TPT (mg/mg protein) | FAT/TPT (%) |
| Control (n = 6) | 0.394±0.094 | 8.90±1.14 | 4.8±1.3 |
| Untreated AP (n = 10) | 1.542±0.249 | 9.77±0.94 | 18.4±3.8b |
| AP+LU 302872 10 mg/kg (n = 6) | 1.543±0.134 | 10.56±1.04 | 14.9±1.2b |
| AP+LU 302872 20 mg/kg (n = 6) | 1.245±0.176 | 9.06±2.18 | 16.7±2.6b |
| AP+LU 302146 10 mg/kg (n = 6) | 0.969±0.08 | 6.61±0.98 | 16.0±2.6b |
| AP+LU 302146 20 mg/kg (n = 6) | 1.869±0.52 | 8.11 ±1.55 | 21.8±3.0b |
Caerulein-induced pancreatitis is a well established and commonly used model of mild, edematous pancreatitis[13,18-21]. In the light microscopy, it displayed features of swelling, inflammatory infiltration composed mainly of neurophils, distinct vacuolization of acinar cells and sporadically necrosis. In our study, the treatment with ET-1 receptor antagonists (non-selective ETA/B and selective ETA) increased slightly the vacuolization of acinar cells but did not affect meaningfully the edema. ET-1 receptor antagonists did not diminish the score of necrosis and it was even slightly increased after both ETA antagonist doses. Parallel, ETA antagonist decreased PMNs infiltration of pancreatic tissue in histological examination. The ultrastructural changes such as decreased number of zymogen granules, disorganization of endoplasmic reticulum, autophagosomes and cytoplasmic vacuoles were even more prominent in treated groups than in rats with untreated AP. The simultaneous biochemical assays revealed that the treatment with non-selective ETA/B and selective ETA antagonists did not affect the increased trypsinogen activation index in pancreatic homogenate as compared to untreated AP.
The overall outcome of the present study was rather surprising for us. In our previous study[8], the same ET-1 receptor antagonists considerably reduced the extent of morphological changes in the pancreas, evaluated by light and electron microscopy in the model of severe AP induced by sodium taurocholate retrograde injection lasting 24 h. Our previous results also suggested a significant role of the antagonism of ET-1 receptors, mainly of the receptor A, which is blocked by the two antagonists, in the inhibition of trypsinogen activation[8]. However, actually in a mild caerulein-induced pancreatitis, we observed no beneficial effects of the same antagonists of ET-1 receptor. This leads us to thinking that the endothelin-1 receptor antagonists may act in different ways in different models of AP. The reason for this is not clear, but can be at least partly explained by differences in pancreatic microcirculation disorders between necrohemorrhagic and edematous AP[9,22]. In severe necrotic pancreatitis, the pancreatic blood flow decreases to 47% of baseline within 6 h[23], and plasma ET-1 level significantly increases[24]. In this situation, application of ET-1 receptor antagonists could considerably improve the circulatory parameters[2-4,10,24] preventing the development of more advanced morphological and biochemical changes. However, the pancreatic capillary flow in edematous, caerulein-induced AP rapidly increases to 188% of baseline and remains elevated up to 6 h of experiment[23], and ET-1 plasma level does not increase[13]. Complete capillary stasis develops in 38% of capillaries in severe AP and is absent in edematous AP[23]. Thus, both the vasoconstrictive action of ET-1 and the blockade of its receptors may be of less importance in the course of edematous AP than in the severe, necrotic one. These differences could be crucial for the explanation of the controversial role of ET-1 receptor antagonists in AP reported in the literature.
Acinar cell vacuolization is a major morphological change in caerulein-induced AP. Watanabe et al[20] have shown that in some developmental stages of this model of disease, vacuoles contain digestive and lysosomal enzymes, which may lead to intracellular activation of trypsinogen by lysosomal cathepsin B and may be an important step in the development of AP. Simultaneous blockade of apical secretion is a prerequisite to basolateral output of partially activated zymogens into the interstitial space with a consecutive release of cytokines and vasoactive mediators, causing edema and chemoattraction of inflammatory cells and activation of vascular endothelium[25]. In our study, we found almost four-fold increase in trypsinogen activation index in pancreatic homogenate in the untreated AP group, compared to control animals. Application of endothelin-1 receptors antagonists had no significant effect on this augmented trypsinogen activation index, as compared to untreated AP nor did it affect meaningfully the edema score. However, the treatment with the selective receptor A antagonist at a dose of 20 mg/kg, slightly but significantly, decreased inflammatory infiltration. According to Plusczyk et al[7], the selective inhibition of the ETA receptor prevents migration of leukocytes in acute experimental pancreatitis. This is consistent with the finding that ETA receptor antagonists inhibit ET-1-mediated adhesion and migration of leukocytes[13,26,27]. Martignoni et al[11] revealed that after the application of selective ETA antagonist (LU 135252), pancreatic edema in animals with caerulein-induced pancreatitis, was similar to that observed in non-treated animals. An increase in edema and in inflammatory infiltration after the administration of BQ-123, a potent ETA receptor antagonist was observed by Kogire et al[13] in caerulein-induced AP. Also Liu et al[6] found no advantageous effect of BQ-123 on swelling and inflammatory infiltration in caerulein+stress-induced pancreatitis, although this antagonist was found to decrease vacuolization and necrosis of acinar cells.
Except for our previous work[8], no reports are available on the ultrastructural changes in pancreatic acinar cells in association with the application of endothelins or ET-1 receptor antagonists in AP. In the group of animals with untreated AP, we observed degenerated acinar cells with vacuoles, dilated rough endoplasmic reticulum, decreased number of zymogen granules and necrosis of some acinar cells. These findings are in accordance with observations of other authors[13,18-20,28,29 ]. The application of ETA/B and ETA receptors antagonists did not improve the ultrastructural picture; on the contrary, it intensified to some degree the changes, especially in the group treated with higher dose of selective ETA antagonist many acinar cells displayed numerous autophagous vacuoles and total cytoplasm disintegration, leading to cell degradation. This group showed the highest trypsinogen activation index, which may confirm the involvement of trypsin in the development and intensification of cellular changes in AP.
Thus, our present examinations, i.e., light microscopy, ultrastructural visualizing of cell organelles and measurement of trypsinogen activation index in pancreatic homogenate, reveal that the treatment with ET-1 receptor antagonists (non-selective ETA/B and selective ETA) does not affect essentially either the edema and inflammatory infiltration or trypsinogen activation observed in the early course of caerulein-induced AP. Nevertheless, a slight increase of the necrosis and vacuolization score and some of the ultrastructural data could suggest the possibility of their undesired effects in caerulein-induced AP at investigated doses. Therefore, it is tempting to suggest that some intracellular effects mediated by ET-1 receptors could be protective in caerulein-induced AP.
LU 302872 is a derivative of endothelin A selective receptor antagonist LU 135252, which retains affinity for ETA but exhibits substantial ETB affinity as well. It antagonises the big ET-induced blood pressure increase in rats and big ET-induced bronchospasm in guinea pigs at the dose of 10 mg/kg[30,31] . LU 302146 is a derivative of LU 135252 with a higher ET-A receptor specificity[32] . Both antagonists have been used at the dose of 30 mg/kg daily p.o., to prevent experimental uremic cardiomyopathy[33]. There are no dates on the doses of used antagonists to be sufficient for complete blockade of ET-1 receptors. The doses of both antagonists chosen in this study are comparable to that in other studies[30,31]. Nevertheless, similar effects of both investigated doses suggest that lower dose (10 mg/kg) is sufficient for the suturing of ET-1 receptors. Different potency or specificity of the endothelin receptor antagonists, dosing differences, the type of animals used and variation in pancreatitis model might be an explanation for the contradictory results reported in the literature.
In conclusion, we are of the opinion that the beneficial effect of endothelin-1 receptor blockade in early, edematous AP could be questioned and the attempts intending to introduce endothelin receptor antagonists to prevent the development of edematous pancreatitis (i.e., socalled post-ERCP pancreatitis) or its transition into necrotic one, should be treated with considerable caution.
Co-first-authors: Anna Andrzejewska and Jan W. Dlugosz
| 1. | Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ, Foitzik T. Effect of endothelin and endothelin receptor blockade on capillary permeability in experimental pancreatitis. Gut. 2000;46:390-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Eibl G, Buhr HJ, Foitzik T. Therapy of microcirculatory disorders in severe acute pancreatitis: what mediators should we block? Intensive Care Med. 2002;28:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Foitzik T, Eibl G, Buhr HJ. Therapy for microcirculatory disorders in severe acute pancreatitis: comparison of delayed therapy with ICAM-1 antibodies and a specific endothelin A receptor antagonist. J Gastrointest Surg. 2000;4:240-246; discussion 247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Foitzik T, Faulhaber J, Hotz HG, Kirchengast M, Buhr HJ. Endothelin mediates local and systemic disease sequelae in severe experimental pancreatitis. Pancreas. 2001;22:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Foitzik T, Hotz HG, Eibl G, Hotz B, Kirchengast M, Buhr HJ. Therapy for microcirculatory disorders in severe acute pancreatitis: effectiveness of platelet-activating factor receptor blockade vs. endothelin receptor blockade. J Gastrointest Surg. 1999;3:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Liu X, Nakano I, Ito T, Kimura T, Nawata H. Is endothelin-1 an aggravating factor in the development of acute pancreatitis? Chin Med J (Engl). 1999;112:603-607. [PubMed] [Cited in This Article: ] |
| 7. | Plusczyk T, Witzel B, Menger MD, Schilling M. ETA and ETB receptor function in pancreatitis-associated microcirculatory failure, inflammation, and parenchymal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G145-G153. [PubMed] [Cited in This Article: ] |
| 8. | Andrzejewska A, Dlugosz JW. The endothelin-1 receptor antagonists ameliorate histology and ultrastructural alterations in the pancreas and decrease trypsinogen activation in severe taurocholate pancreatitis in rats. Int J Exp Pathol. 2003;84:221-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Todd KE, Lewis MP, Gloor B, Lane JS, Ashley SW, Reber HA. An ETa/ETb endothelin antagonist ameliorates systemic inflammation in a murine model of acute hemorrhagic pancreatitis. Surgery. 1997;122:443-449; discussion 449-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Foitzik T, Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ. Endothelin receptor blockade in severe acute pancreatitis leads to systemic enhancement of microcirculation, stabilization of capillary permeability, and improved survival rates. Surgery. 2000;128:399-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Martignoni ME, Ceyhan GO, Ayuni E, Kondo Y, Zimmermann A, Büchler MW, Friess H. Endothelin receptor antagonists are not beneficial in the therapy of acute experimental pancreatitis. Langenbecks Arch Surg. 2004;389:184-192. [PubMed] [Cited in This Article: ] |
| 12. | Fiedler F, Ayasse D, Rohmeiss P, Gretz N, Rehbein C, Keim V. The endothelin antagonist bosentan does not improve survival in severe experimental pancreatitis in rats. Int J Pancreatol. 1999;26:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kogire M, Inoue K, Higashide S, Takaori K, Echigo Y, Gu YJ, Sumi S, Uchida K, Imamura M. Protective effects of endothelin-1 on acute pancreatitis in rats. Dig Dis Sci. 1995;40:1207-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Yamaguchi H, Kimura T, Mimura K, Nawata H. Activation of proteases in cerulein-induced pancreatitis. Pancreas. 1989;4:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kyogoku T, Manabe T, Tobe T. Role of ischemia in acute pancreatitis. Hemorrhagic shock converts edematous pancreatitis to hemorrhagic pancreatitis in rats. Dig Dis Sci. 1992;37:1409-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Dlugosz JW, Wroblewski E, Poplawski C, Gabryelewicz A, Andrzejewska A. Does antecedent ethanol intake affect course of taurocholate pancreatitis in rats? Dig Dis Sci. 1997;42:944-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Andrzejewska A, Dlugosz JW, Jurkowska G. The effect of antecedent acute ethanol ingestion on the pancreas ultrastructure in taurocholate pancreatitis in rats. Exp Mol Pathol. 1998;65:64-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 409] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Takano S, Kimura T, Kawabuchi M, Yamaguchi H, Kinjo M, Nawata H. Ultrastructural study of the effects of stress on the pancreas in rats. Pancreas. 1994;9:249-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457-G467. [PubMed] [Cited in This Article: ] |
| 21. | Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933-936. [PubMed] [Cited in This Article: ] |
| 22. | Klar E, Schratt W, Foitzik T, Buhr H, Herfarth C, Messmer K. Impact of microcirculatory flow pattern changes on the development of acute edematous and necrotizing pancreatitis in rabbit pancreas. Dig Dis Sci. 1994;39:2639-2644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Schmidt J, Ebeling D, Ryschich E, Werner J, Gebhard MM, Klar E. Pancreatic capillary blood flow in an improved model of necrotizing pancreatitis in the rat. J Surg Res. 2002;106:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Foitzik T, Faulhaber J, Hotz HG, Kirchengast M, Buhr HJ. Endothelin receptor blockade improves fluid sequestration, pancreatic capillary blood flow, and survival in severe experimental pancreatitis. Ann Surg. 1998;228:670-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Klar E, Werner J. New pathophysiologic knowledge about acute pancreatitis. Chirurg. 2000;71:253-264. [PubMed] [Cited in This Article: ] |
| 26. | Cui P, Tani K, Kitamura H, Okumura Y, Yano M, Inui D, Tamaki T, Sone S, Kido H. A novel bioactive 31-amino acid endothelin-1 is a potent chemotactic peptide for human neutrophils and monocytes. J Leukoc Biol. 2001;70:306-312. [PubMed] [Cited in This Article: ] |
| 27. | Zouki C, Baron C, Fournier A, Filep JG. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: role of ET(A) receptors and platelet-activating factor. Br J Pharmacol. 1999;127:969-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Brunelli A, Scutti G. An ultrastructural study to investigate the effect of allopurinol on cerulein-induced damage to pancreatic acinar cells in rat. Int J Pancreatol. 1998;23:25-29. [PubMed] [Cited in This Article: ] |
| 29. | Grönroos JM, Aho HJ, Hietaranta AJ, Nevalainen TJ. Early acinar cell changes in caerulein-induced interstitial acute pancreatitis in the rat. Exp Pathol. 1991;41:21-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Amberg W, Hergenröder S, Hillen H, Jansen R, Kettschau G, Kling A, Klinge D, Raschack M, Riechers H, Unger L. Discovery and synthesis of (S)-3-[2-(3,4-dimethoxyphenyl)ethoxy]-2- (4,6-dimethylpyrimidin-2-yloxy)-3,3-diphenylpropionic acid (LU 302872), a novel orally active mixed ET(A)/ET(B) receptor antagonist. J Med Chem. 1999;42:3026-3032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Raschack M, Göck S, Unger L, Hahn A, Amberg W, Jansen R, Alken P, Weber A, Hergenröder S. LU 302 872 and its racemate (LU 224 332) show balanced endothelin-A/B receptor affinity, high oral activity, and inhibit human prostate tissue contractions. J Cardiovasc Pharmacol. 1998;31 Suppl 1:S241-S244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Knoll T, Oltersdorf J, Göttmann U, Schaub M, Michel MS, Kirchengast M, van der Woude FJ, Rohmeiss P, Braun C. Influence of acute selective endothelin-receptor-A blockade on renal hemodynamics in a rat model of chronic allograft rejection. Transpl Int. 2003;16:425-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Wolf SC, Amend T, Risler T, Amann K, Brehm BR. Influence of endothelin receptor antagonists on myocardial protein kinase C isoforms in uraemic cardiomyopathy. Clin Sci (Lond). 2002;103 Suppl 48:276S-279S. [PubMed] [Cited in This Article: ] |