Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3091
Revised: December 23, 2004
Accepted: January 5, 2005
Published online: May 28, 2005
AIM: Gastro-esophageal reflux disease (GERD) is becoming increasingly common in Asia. Data on the efficacy of proton pump inhibitors in patients with non-erosive GERD (NERD) in Asia is lacking. This double-blind study compared the efficacy and safety of rabeprazole with esomeprazole in relief of symptoms in patients with NERD.
METHODS: One hundred and thirty-four patients with reflux symptoms of NERD and normal endoscopy were randomized to receive rabeprazole 10 mg or esomeprazole 20 mg once daily for 4 wk. Symptoms were recorded in a diary and changes in severity of symptoms noted.
RESULTS: At 4 wk of treatment, rabeprazole 10 mg and esomeprazole 20 mg were comparable with regards to the primary endpoint of time to achieve 24-h symptom-free interval for heartburn 8.5 d vs 9 d and regurgitation 6 d vs 7.5 d. Rabeprazole and esomeprazole were also similarly efficacious in term of patient’s global evaluation with 96% of patients on rabeprazole and 87.9% of patients on esomeprazole, reporting that symptoms improved (P = NS). Satisfactory relief of day- and night-time symptoms was achieved in 98% of patients receiving rabeprazole and 81.4% of patients receiving esomeprazole. Adverse events were comparable in both groups (P = NS).
CONCLUSION: Rabeprazole 10 mg has a similar efficacy and safety profile in Asians with NERD as esomeprazole 20 mg. Further study is necessary to investigate whether the small differences between the two drugs seen in this study are related to the improved pharmacodynamic properties of rabeprazole. Both drugs were well tolerated.
-
Citation: Fock K, Teo E, Ang T, Chua T, Ng T, Tan Y. Rabeprazole
vs esomeprazole in non-erosive gastro-esophageal reflux disease: A randomized, double-blind study in urban Asia. World J Gastroenterol 2005; 11(20): 3091-3098 - URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3091.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3091
Gastro-esophageal reflux disease (GERD) is characterized by recurrent return of gastric contents back into the esophagus, causing heartburn, regurgitation and symptoms, such as chest pain, coughing, hoarseness and dysphagia[1,2]. GERD symptoms can cause significant patient distress and can interfere with everyday life[2].
GERD is a common condition, with an estimated 44% of the adult population in USA experiencing GERD symptoms monthly and about 20% experiencing symptoms weekly[1]. However, prevalence of GERD in Asia is reported to be lower than that in Western countries[3]. A study from Singapore in 1988 reported that the prevalence of monthly reflux symptoms in the community was 1.6%[4]. Recently, a study performed in Hong Kong reported that the monthly and weekly prevalence of GERD symptoms was 8.9% and 2.5%, respectively[5].
GERD can be subdivided into several groups: (1) non-erosive GERD (NERD), (2) erosive GERD, (3) Barrett’s esophagus, and GERD-related complications. NERD has been defined as the presence of typical symptoms of GERD caused by intra-esophageal acid in the absence of visible esophageal mucosal injury[1]. In NERD patients the total acid reflux time has been found to be significantly lower than that in patients with erosive esophagitis. Furthermore, as much as 50% of NERD patients have normal 24-h esophageal pH study[6]. It is estimated that up to 70% of patients with typical GERD symptoms in the West have normal endoscopy[7]. In Asia, NERD and endoscopically mild form of erosive esophagitis may account for up to 90% of patients with GERD symptoms[8].
Proton pump inhibitors (PPIs), such as omeprazole, esomeprazole, lansoprazole and rabeprazole, are widely used for the treatment of GERD. PPIs effectively inhibit the duration and extent of gastric acid secretion and provide more complete remission of the symptoms of heartburn than other forms of acid suppression therapy[9,10]. However, the response to PPI in patients with NERD is less efficacious when compared to patients with erosive GERD[1,11].
The goal of treatment is to improve patients’ quality of life by providing rapid relief of symptoms and reducing the severity and number of recurrent episodes[8]. Therefore, an important endpoint in clinical trials assessing the efficacy of treatment in NERD patients is time taken for complete relief of symptoms, especially the pivotal symptoms of heartburn and regurgitation[8]. This can be measured as time to the first 24-h interval free from GERD/NERD symptoms of heartburn or acid regurgitation. Other endpoints include global symptom improvement, satisfactory, and complete relief of day- and night-time symptoms.
Two new PPIs have been introduced in Asia recently: rabeprazole and esomeprazole. Rabeprazole is a PPI that effectively provides symptom relief and healing, and prevents relapse, in patients with erosive GERD[12-14]. One clinical study suggests that rabeprazole effectively relieves the symptoms of heartburn in patients with NERD with significant improvement starting with the dose on 1st d[11]. Esomeprazole, the s-enantiomer of omeprazole, has demonstrated superior efficacy over omeprazole in healing and symptom resolution in patients with erosive and non-erosive reflux disease[15,16].
Currently, there is a paucity of clinical data comparing the efficacy and safety of these two PPIs in treating NERD patients let alone in Asian population. We report a randomized, double-blind, parallel-group, 4-wk study designed to investigate the efficacy and safety of rabeprazole 10 mg compared with esomeprazole 20 mg once daily in the treatment of NERD patients. This is the first clinical study directly comparing the efficacy of these two PPIs in NERD patients in Asia.
The study was conducted in accordance with the Declaration of Helsinki. Ethics committee approval was obtained before the study commenced and all patients gave informed consent.
In this randomized, double-blind, parallel-group comparative study, patients with NERD received rabeprazole 10 mg once daily or esomeprazole 20 mg once daily for a 4-wk treatment period. Patients recorded their GERD symptoms (heartburn or regurgitation, with or without eructation) daily in the diary provided. Other upper GI symptoms were similarly recorded as well. Patients were screened 7 d prior to enrollment and eligibility was assessed according to the specified inclusion and exclusion criteria. The study consisted of a 1-wk screening phase, followed by endoscopy and a 4-wk, double-blind treatment phase. Helicobacter pylori screening was performed using CLO-test and serology.
To be eligible for study entry, patients were required to be aged between 21 and 65 years. GERD symptoms (i.e., heartburn or regurgitation or both) were dominant symptoms. Heartburn or regurgitation was present for at least 3 mo in the previous year, which need not be continuous.
Heartburn was defined as ‘substernal burning sensation or pain’. A description like ‘a burning sensation behind the breastbone rising up to the throat or neck’ or ‘a burning pain or discomfort behind the breastbone rising up towards the neck’ was accepted as ‘heartburn’. Patients who described these symptoms as ‘a burning, warm or ‘acid’ sensation in the epigastrium, substernal area or both’ were also accepted as having ‘heartburn’. Regurgitation was defined as ‘food or fluid coming back up from your stomach’. Eructation was defined as ‘belching’.
To qualify for inclusion into the study, subjects need to have experienced at least one period of moderate-to-very severe heartburn or regurgitation in the past 7 d prior to treatment.
In addition, at endoscopy, no esophageal mucosal break was observed, i.e., grade 0 according to the LA Classification. The ability to read and write in either English or Chinese was also a requirement for study entry.
The main exclusion criteria were as follows: known history of gastroduodenal ulcer; infectious or inflammatory conditions of the intestine (including inflammatory bowel disease); malabsorption syndromes; obstruction; gastrointestinal malignancy; gastric or intestinal surgery including vagotomy; Barrett’s esophagus; esophageal stricture or pyloric stenosis; scleroderma; erosive esophagitis; positive HIV status and pregnancy. Patients were ineligible if they had: abnormal laboratory tests at the initial visit (including liver enzymes greater than twice the upper limit of normal); GERD treatment refractory to a 2-mo course of H2-blocker or PPI therapy; taken a PPI within 14 d of screening or a H2-blocker or prokinetic agent within 7 d of screening; required daily use of NSAIDS, oral steroids, aspirin (>325 mg/d); or were unable to discontinue the use of anticholinergics, cholinergics, spasmolytics, opiates or sucralfate.
Patients who qualified were randomized to receive either rabeprazole 10 mg or esomeprazole 20 mg once daily after the morning meal. A computer-generated randomization scheme was used to randomly allocate patients to one of the two treatment groups.
Patients were permitted to take an antacid (Mylanta®) as rescue medication for the relief of heartburn symptom, if necessary. No other medication was allowed.
Rabeprazole 10-mg tablets and esomeprazole 20-mg tablets were inserted into identical capsules to ensure double blinding. Patients received 2 wk supply of medication at each study visit.
The study consisted of a screening visit and three scheduled visits during the treatment phase: baseline (the end of the screening phase), wk 2 and 4. At each visit, a review of concurrent and disallowed medications was undertaken, completed patient diaries were collected and adverse events were assessed. An upper gastrointestinal endoscopy was conducted 7 d prior to baseline or at the baseline visit. Laboratory analysis was conducted at baseline and at wk 4. Compliance with drug therapy was determined at the scheduled wk 2 and 4 visits.
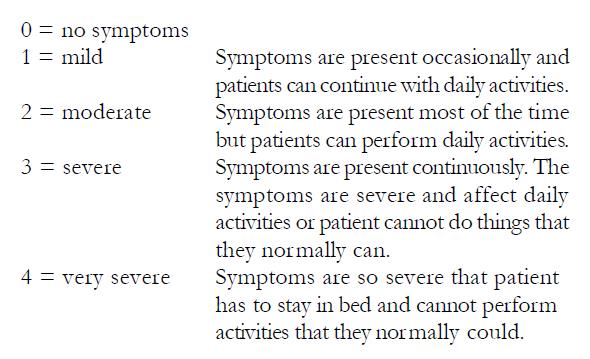
Patients recorded the severity of GERD symptoms in a daily diary. Severity was graded on a five-point scale from none (0), mild (1), moderate (2), severe (3) and very severe (4) for each of the following symptoms: day-time heartburn, night-time heartburn, day-time regurgitation, and night-time regurgitation.
Other upper GI symptoms of belching (‘eructation’), early satiety (‘the sensation of filling up quickly’), bloating (‘feeling like I have a lot of gas in my belly’), nausea and vomiting were also recorded on the five-point scale as explained above.
The symptom severity was defined in Math 1.
Math 1
The Patient Informed Consent was in English; however, identical versions translated into Malay and Mandarin were available to the patients as well. In the development of the Malay and Mandarin versions of the Patient Informed Consent, the original English version was translated, back-translated and checked for accuracy. The Patient Informed Consent was explained in English, Malay or Mandarin according to the subject’s first language or preferred language of communication.
The primary efficacy endpoint was the time (in days) for patients to achieve their first 24-h interval without any symptoms of heartburn or regurgitation. Secondary endpoints were as follows: number of patients who had complete or satisfactory relief of symptoms during wk 1, 2, 3, or 4, symptom severity scores of day-time and night-time heartburn or regurgitation, upper GI symptoms, patients’ global evaluation at the end of study and number of antacids used during the study period.
Safety and tolerability were evaluated by recording adverse events (including severity, relationship of the adverse event to the study treatment and outcome and laboratory analysis).
The Case Report Forms were available in English, Malay and Mandarin, according to the subject’s first language or preferred language of communication. In the development of the Malay and Mandarin identical versions of the Case Report Form, similar care was taken: the original English version was translated, back-translated and checked for accuracy.
Analyses were performed on an intention-to-treat (ITT) basis by an independent statistician. The ITT population was defined as including all randomized patients who received at least one dose of study medication and who had at least one post-baseline assessment for efficacy. The primary outcome, i.e., time taken to achieve 24-h symptom free from heartburn or regurgitation, however, did not include for analysis of patients who did not experience heartburn and/or regurgitation on the day prior to commencement of study medication. Heartburn and regurgitation were analyzed separately.
Subgroup analyses were performed for the subjects who experienced heartburn and/or regurgitation.
Day-time symptoms were those that occur after arising in the morning. Night-time symptoms were those that occur after retiring in the evening. Multiple single episodes experienced during a day-time and/or a night-time period count only as 1 d-time and/or 1 night-time episode.
Differences within or between treatment groups for all tests were considered significant at P≤0.05.
In order to detect a difference in clinical response of 20% or more between the two treatment groups with the use of a two-sided test with 0.80 statistical power and a significant level of 0.05, a sample size of 118 was required. Hence the sample size was determined to be 130, with an allowance of 10% for patients who were lost to follow up. A magnitude of 20% was chosen on the basis that it represented a clinically relevant difference in outcome.
Student’s t-test and Fischer’s exact test were used to compare the patient demographics of the two groups of patients. Subject global evaluation was analyzed using Wilcoxon’s test. The primary efficacy parameter was analyzed using log-rank test. The percentage of patients experiencing complete and satisfactory relief of heartburn and regurgitation during the study (day-time and night-time) was analyzed using repeated measurement analysis. The average reflux symptom scores were analyzed using an analysis of covariance (ANCOVA) model between the two PPIs and using paired t-test when analyzed between treatment and pre-treatment (baseline). The average weekly antacid tablets consumed were analyzed using an ANCOVA model. The percent of periods without antacids consumption were analyzed using analysis of variance model. Analysis of laboratory data was compared using paired t-tests.
Withdrawal from the study was allowed in the event of a serious adverse event, the detection of intercurrent illness that might invalidate the study or place the patient at risk, concern for patient safety by the investigator, protocol violations or unreliable patient behavior.
One hundred and thirty-four patients were enrolled (67 from each treatment group) in the study and randomly assigned to receive either rabeprazole 10 mg or esomeprazole 20 mg. There were 63 patients in the rabeprazole treatment group and 64 patients in the esomeprazole treatment group with a total of 127 patients eligible for efficacy analysis (ITT)(Table 1). Of the seven patients in total excluded from the efficacy analysis (ITT), there were four patients in the rabeprazole and three patients in the esomeprazole group. Of these, four rabeprazole patients and one esomeprazole patient did not take any study medication. One esomeprazole patient withdrew due to persistent headache and another esomeprazole patient withdrew consent after taking study medication for 2 d. Although these latter two patients on esomeprazole did receive at least one dose of study medication, they did not have at least one post-baseline assessment for efficacy (as defined and required in the protocol for ITT analysis). Therefore, they were not included in the ITT analysis for efficacy.
| Number of subjects enrolled | Total 127 | Rabeprazole 63 | Esomeprazole 64 | P |
| Gender (%) | ||||
| Female | 62 (48.8) | 25 (39.7) | 37 (57.8) | P = 0.0511 |
| Male | 65 (51.2) | 38 (60.3) | 27 (42.2) | |
| Race (%) | ||||
| Chinese | 101 (79.5) | 52 (82.5) | 49 (76.6) | P = 0.8721 |
| Malay | 9 (7.1) | 4 (6.3) | 5 (7.8) | |
| Indian | 15 (11.8) | 6 (9.5) | 9 (14.1) | |
| Other | 2 (1.6) | 1 (1.6) | 1 (1.6) | |
| Age (yr) | ||||
| Mean (SD) | 38.9 (10.6) | 39.3 (11.2) | 38.4 (10.0) | P = 0.6292 |
| History of GERD symptoms (yr) | ||||
| Mean (SD) | 3.6 (4.5) | 3.2 (4.2) | 3.9 (4.7) | P = 0.3732 |
| Tobacco use, N (%) | ||||
| Yes | 11 (8.7) | 4 (6.3) | 7 (10.9) | P = 0.2431 |
| No | 116 (91.3) | 59 (93.7) | 57 (89.1) | |
| Alcohol use, N (%) | ||||
| Yes | 20 (15.7) | 9 (14.3) | 11 (17.2) | P = 0.4861 |
| No | 107 (84.3) | 54 (85.7) | 53 (82.8) | |
| Previous medication for reflux disease | ||||
| Yes | 77 (60.6) | 35 (55.6) | 42 (65.6) | P = 0.2791 |
| No | 50 (39.4) | 28 (44.4) | 22 (34.4) | |
| H pylori status | ||||
| Positive | 50 | 24 (45.3) | 26 (44.0) | |
| Negative | 62 | 29 (54.7) | 33 (56.0) | P = 0.953 |
| (Not available | 15 | 10 | 5 |
The treatment groups were similar with respect to demographic and clinical characteristics. Although there were more males in the rabeprazole group, this difference was not statistically significant. The mean age of study participants was 38.9 years. The majority of patients were of Chinese descent (79.5%, Table 1).
A summary of the efficacy (and safety) results can be seen in Table 2.
| Parameter | Rabeprazole 10 mg (d) | Esomeprazole 20 mg (d) | P | Result |
| Primary efficacy variables | ||||
| Time to 24-h symptom-free interval-HB | 8.5 d | 9 d | 0.265 | NS |
| Time to 24-h symptom-free interval-RG | 6.0 d | 7.5 d | 0.405 | NS |
| Secondary efficacy variables | ||||
| Time to 48-h symptom-free interval-HB | 9.5 d | 8.5 d | 0.373 | NS |
| Time to 48-h symptom-free interval-RG | 8.5 d | 11 d | 0.271 | NS |
| W1-W4-satisfactory relief DT or NT-HB | >0.05 | NS | ||
| W1-W4-satisfactory relief DT or NT-RG | >0.05 | NS | ||
| W1-W4-satisfactory relief DT-HB & RG4 | 0.0454 | Rabeprazole superior4 | ||
| W1-W4-complete relief DT or NT-HB | >0.05 | NS | ||
| W1-W4-complete relief DT or NT-RG | >0.05 | NS | ||
| W1-W4-belching | -0.41 | -0.42 | 0.631 | NS |
| W1-W4-early satiety | -0.26 | -0.32 | 0.178 | NS |
| W1-W4-bloating | -0.46 | -0.54 | 0.608 | NS |
| W1-W4-nausea | -0.23 | -0.27 | 0.319 | NS |
| W1-W4-vomiting | -0.34 | -0.21 | 0.808 | NS |
| Symptom severity score-D1-5-DT HB | P<0.05 (D2-5)1 | P<0.05 (D3-5)1 | NS2 | |
| Symptom severity score-D1-5-NT HB | P<0.05 (D2-5)1 | NS1 | NS3 | |
| Symptom severity score-D1-5-DT RG | P<0.05 (D1-5)1 | P<0.05 (D1-5)1 | NS | |
| Symptom severity score-D1-5-NT RG | P<0.05 (D5 only)1 | P<0.05 (D2 only)1 | NS | |
| Patient’s global evaluation (%) | 96.4 | 87.9 | 0.823 | NS |
| Antacid use-weekly average | 0.15 | 0.16 | 0.887 | NS |
| Antacid use-% antacid free | 85.7 | 84.9 | 0.848 | NS |
| Safety | ||||
| Adverse events | 22 | 18.2 | >0.05 | NS |
Time to first 24-h, symptom-free interval The median time to the first 24-h symptom-free interval was similar for patients in both treatment groups; 8.5 d for rabeprazole and 9.0 d for esomeprazole for heartburn (P = NS) and 6.0 d vs 7.5 d for regurgitation (P = NS). The proportion of patients achieving these study endpoints during the 4-wk treatment period were higher in rabeprazole group compared with esomeprazole group, but these differences were not statistically significant (24-h heartburn-free for rabeprazole and esomeprazole: 84.4% vs 60.9%, 24-h regurgitation-free: 90.0% vs 67.9% (P = NS).
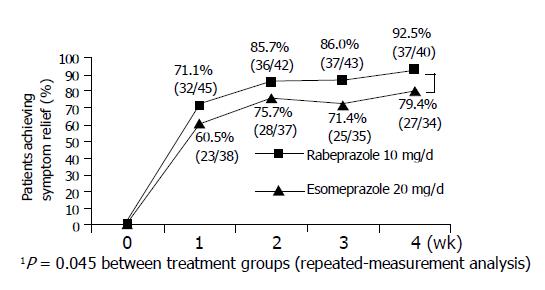
Satisfactory relief of day-time and night-time symptoms Satisfactory relief of day-time and night-time symptoms (no episode of symptom defined as having moderate or severe in severity during the week) was achieved in 81.4-98.0% of patients of both treatment groups of heartburn or regurgitation after 4-wk treatment.
In a subgroup of patients who had both heartburn and regurgitation, a statistically significant higher number of patients treated with rabeprazole reported satisfactory relief of day-time symptoms compared to those receiving esomeprazole (P<0.05, Figure 1).
Complete relief of day-time heartburn (no episodes of heartburn during the evaluation week) at the 1st wk were 6.9% (14 of 52 patients) in patients treated with rabeprazole and 23.4% (11 out of 52 patients) in those received esomeprazole (P = NS). At the end of wk 4, this increased to 55.3% (26 out of 47) and 41.1% (18 out of 43) for rabeprazole and esomeprazole, respectively (P = NS). Complete relief of night-time heartburn were similar in both patient groups (28.8% (15/62) vs 20.9% (9/43)) at wk 1 and (44.4% (20/45) vs 41.0% (16/39)) at wk 4 (for rabeprazole and esomeprazole, respectively) (P = NS).
No statistically significant differences were observed in analyses of regurgitation.
Comparing between the two PPIs, there was no statistically significant difference between groups for day- and night-time heartburn or regurgitation within the first 5 d (P = NS, Table 2).
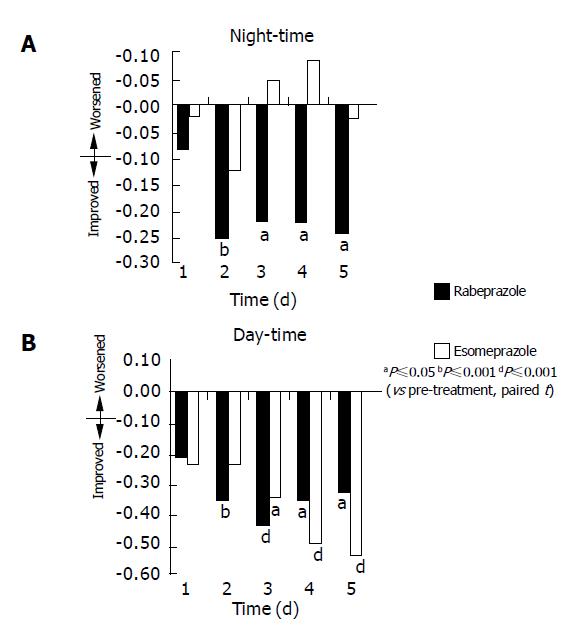
Comparing each individual PPI with treatment vs baseline or pre-treatment symptoms severity scores, rabeprazole significantly reduced day- and night-time heartburn scores within 2 d of commencing treatment compared to baseline or pre-treatment (P<0.01), and this statistical significance continued up to d 5. However, patients receiving esomeprazole showed a statistically significant improvement in day-time heartburn score from the 3rd to the 5th d, and no significant improvement in night-time heartburn score in the first 5 d (Table 2 and Figure 2).
The use of rescue medication was low in both groups. There was no statistical difference between the two groups for weekly average antacid consumption and percentage antacid free for the duration of the study (P = NS).
A slightly higher proportion of patients treated with rabeprazole (96.4%, 54 out of 56 patients) reported their symptoms as improved (“slightly improved”, “moderately improved” or “markedly improved”) at the end of the treatment period compared to those treated with esomeprazole (87.9%, 51 out of 58 patients). The difference was not statistically significant.
Of the 134 patients enrolled, 129 patients in total were eligible for safety analysis. The five patients excluded from the safety analysis did not take any study medication. Of the five patients, four were from the rabeprazole and one was from the esomeprazole group.
Both drugs were well tolerated during the study. Adverse events considered related to the study medication occurred to a similar extent in patients treated with rabeprazole (22%) and esomeprazole (18.2%) (P = NS). One patient withdrew from the study because of persistent headache from esomeprazole.
Elevation of ALT occurred in one patient taking rabeprazole and four patients receiving esomeprazole. One patient on rabeprazole and two patients on esomeprazole had an increase in AST. These changes were not clinically significant.
There were no obvious differences in tolerability between the treatments. The measurement of laboratory variables and vital signs did not reveal any evidence of deleterious effects of the drugs in either group.
One of the problems in defining GERD in Asia is that there is no direct translation of ‘heartburn’ in most Asian languages, including the Chinese language[7,8] although this gap has been closed since the Asia-Pacific Consensus. Accepted classic symptoms of GERD in Asia include heartburn, regurgitation, dysphagia and odynophagia[7]. The recent Asia-Pacific Consensus on GERD recommends that a ‘careful history taking to elicit the classic symptoms of GERD (heartburn and regurgitation) is the cornerstone in the diagnosis of GERD’[8]. In the present study, every effort was made by the investigating physician to ensure that heartburn and/or regurgitation was/were the cardinal presenting symptom(s). In addition, care was taken by the investigators to explain and elicit what the symptom of ‘heartburn’ meant to the patient. This study was designed to assess the efficacy and rapidity of symptom relief with rabeprazole 10 mg or esomeprazole 20 mg in patients with NERD in urban Asia.
The primary efficacy variable of median time to the first 24-h interval free from heartburn was similar for both drugs being 8.5 d for rabeprazole and 9.0 d for esomeprazole. Results of the primary efficacy variable for relief of regurgi-tation were similar between the two PPIs as well.
The majority of secondary efficacy variables were similar between the two PPIs as well. Overall, after 4 wk of treatment more patients receiving rabeprazole reported symptom improvement than those receiving esomeprazole (96% vs 87%) although this did not reach statistical significance. Complete relief of day-time symptoms after 4 wk of treatment was reported in 41.9% and 58.0%, whilst complete relief of night-time symptoms was lower at 41.0% vs 52.3%. Satisfactory relief of symptoms, defined as not having any episode greater than moderately severe, was reported in 73% (esomeprazole) to 96% (rabeprazole) of patients treated. In real-life situation, most patients would find satisfactory relief of symptoms as an acceptable treatment outcome. Further studies, however, are needed to confirm these observations. Our observations do support the clinical phar-macokinetics studies currently available on the two PPIs.
Pharmacodynamic studies involving rabeprazole and esomeprazole in healthy subjects showed that 40 mg of esomeprazole was more effective than 20 mg rabeprazole on d 5 of treatment in maintaining intragastric pH above 4.0, suggesting that, by d 5, 40 mg esomeprazole had more profound acid suppression than 20 mg rabeprazole[17]. On the other hand, 20 mg of rabeprazole increased intragastric pH more than 20 mg esomeprazole with a higher mean AUC (area under the plasma concentration-time curve) intragastric pH on d 1 of treatment[18]. On d 5 that difference remained except 11-14 h after dosing[18]. A study comparing rabeprazole 20 mg and esomeprazole 40 mg demonstrated rabeprazole 20 mg to produce a greater or equivalent acid suppression on day 1 (i.e., from the first dose), with rabeprazole showing significant superiority at night[19,20]. These studies demonstrate that rabeprazole have a faster onset of acid inhibitory action than other PPIs including esomeprazole from d 1 of dosing[18-20], with esomeprazole the superiority is seen over other PPIs from day 5 of dosing[17].
There was no statistically significant difference between the two groups for reduction in symptom severity scores for the first 5 d during day-time and night-time regurgitation. Nevertheless, rabeprazole effectively reduced the severity of day-time and night-time heartburn in patients with NERD compared to pre-treatment, improving symptom scores compared with baseline scores from as early as the 2nd d (P<0.05) following dosing. In contrast, esomeprazole produced significant improvement only in the symptom score of day-time heartburn from 3rd d onwards and no statistically significant change in symptom score in night-time heartburn in the first 5 d.
Several reports have suggested that patients with NERD are less responsive to PPIs[21-23]. Our study showed that after 4-wk treatment, both PPIs produced satisfactory relief of day-time heartburn in 91% of patients with non-erosive reflux disease (91% rabeprazole and 79% esomeprazole). This response rate is higher than that seen in Miner’s study in USA, where only 56% of NERD patients responded to PPI after 4 wk[11]. Complete relief of day-time heartburn was also higher in our study being reported in 45.6% (rabeprazole) and 41% (esomeprazole) of the patients treated compared with 29% in Miner’s study[11]. This could be due to selection of a less severely symptomatic patient population. Patients were required to have had only one episode of moderate-to-severe GERD in the 7 d prior to study entry, whereas entry into the Miner study required patients to have had a minimum of five episodes in the week prior to entry[11]. Nevertheless, there are about 20% of our NERD patients who did not attain satisfactory relief of symptoms after 4 wk of treatment. Our data has shown that more patients respond to PPIs at wk 4 than at wk 1. Hence, by extending the duration of therapy beyond 4 wk, it may be possible that more patients would have symptom relief. Clinical studies with longer treatment periods are needed to determine if this hypothesis is true. Recent investigations have demonstrated the presence of highly acidic ‘pocket’ high in the fundus below the cardio-esophageal junction during the post-prandial period[24]. Dosing with acid suppressing agents will have to be tailored to neutralize this post-prandial acid ‘pocket’.
Although there was rapid onset of action, the median time to the first 24-h period symptom free from heartburn was 8.5 d with rabeprazole and 9.0 d with esomeprazole. This was higher than the 2.5 d observed in Miner’s study[11]. This difference could be explained by the fact that, in our study, patients who did not experience reflux symptoms 24 h prior to study were excluded from the analysis whereas they were included in Miner’s study[11]. As these patients were excluded from analysis, the median reported in the study would overestimate the actual time taken to reach the first 24-h interval free from heartburn. The rapid action of these newer PPIs could be clinically relevant when treating NERD patients with “on-demand therapy”, as these newer PPIs could produce symptom relief from d 2 or 3 onwards. This would translate into a shorter period of ‘on-demand’ therapy. This is consistent with the results seen in earlier report regarding on-demand treatment with rabeprazole 10 mg[25]. In that study, use of rabeprazole was required in only 26% of the total study period, indicating an average intake of only one tablet in 4 d[25].
In summary, our study demonstrates that once-daily therapy with the newer PPIs rabeprazole 10 mg or esomeprazole 20 mg produce improvement in majority of NERD patients. Relief from the symptoms of heartburn and regurgitation in a predominantly Chinese population with NERD can occur after 2 d of treatment. Further studies are needed to determine the optimum treatment period and symptom relapse rate on cessation of treatment.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Fass R. Epidemiology and pathophysiology of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98:S2-S7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Damiano A, Siddique R, Xu X, Johanson J, Sloan S. Reductions in symptom distress reported by patients with moderately severe, nonerosive gastroesophageal reflux disease treated with rabeprazole. Dig Dis Sci. 2003;48:657-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Holtmann G. Reflux disease: the disorder of the third millennium. Eur J Gastroenterol Hepatol. 2001;13 Suppl 1:S5-11. [PubMed] [Cited in This Article: ] |
| 4. | Ho KY, Kang JY, Seow A. Prevalence of gastrointestinal symptoms in a multiracial Asian population, with particular reference to reflux-type symptoms. Am J Gastroenterol. 1998;93:1816-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 217] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Wong WM, Lai KC, Lam KF, Hui WM, Hu WH, Lam CL, Xia HH, Huang JQ, Chan CK, Lam SK. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: a population-based study. Aliment Pharmacol Ther. 2003;18:595-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non-erosive reflux disease (NERD)--acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003;17:537-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Goh KL, Chang CS, Fock KM, Ke M, Park HJ, Lam SK. Gastro-oesophageal reflux disease in Asia. J Gastroenterol Hepatol. 2000;15:230-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Fock KM, Talley N, Hunt R, Fass R, Nandurkar S, Lam SK, Goh KL, Sollano J. Report of the Asia-Pacific consensus on the management of gastroesophageal reflux disease. J Gastroenterol Hepatol. 2004;19:357-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Holtmann G, Bytzer P, Metz M, Loeffler V, Blum AL. A randomized, double-blind, comparative study of standard-dose rabeprazole and high-dose omeprazole in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:479-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Galmiche JP, Zerbib F, Ducrottè P, Fournet J, Rampal P, Avasthy N, Humphries TJ. Decreasing oesophageal acid exposure in patients with GERD: a comparison of rabeprazole and omeprazole. Aliment Pharmacol Ther. 2001;15:1343-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Miner P, Orr W, Filippone J, Jokubaitis L, Sloan S. Rabeprazole in nonerosive gastroesophageal reflux disease: a randomized placebo-controlled trial. Am J Gastroenterol. 2002;97:1332-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Cloud ML, Enas N, Humphries TJ, Bassion S. Rabeprazole in treatment of acid peptic diseases: results of three placebo-controlled dose-response clinical trials in duodenal ulcer, gastric ulcer, and gastroesophageal reflux disease (GERD). The Rabeprazole Study Group. Dig Dis Sci. 1998;43:993-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Dekkers CP, Beker JA, Thjodleifsson B, Gabryelewicz A, Bell NE, Humphries TJ. Double-blind comparison [correction of Double-blind, placebo-controlled comparison] of rabeprazole 20 mg vs. omeprazole 20 mg in the treatment of erosive or ulcerative gastro-oesophageal reflux disease. The European Rabeprazole Study Group. Aliment Pharmacol Ther. 1999;13:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Thjodleifsson B, Rindi G, Fiocca R, Humphries TJ, Morocutti A, Miller N, Bardhan KD. A randomized, double-blind trial of the efficacy and safety of 10 or 20 mg rabeprazole compared with 20 mg omeprazole in the maintenance of gastro-oesophageal reflux disease over 5 years. Aliment Pharmacol Ther. 2003;17:343-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Kahrilas PJ, Falk GW, Johnson DA, Schmitt C, Collins DW, Whipple J, D'Amico D, Hamelin B, Joelsson B. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther. 2000;14:1249-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Lind T, Rydberg L, Kylebäck A, Jonsson A, Andersson T, Hasselgren G, Holmberg J, Röhss K. Esomeprazole provides improved acid control vs. omeprazole In patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14:861-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Wilder-Smith CH, Röhss K, Nilsson-Pieschl C, Junghard O, Nyman L. Esomeprazole 40 mg provides improved intragastric acid control as compared with lansoprazole 30 mg and rabeprazole 20 mg in healthy volunteers. Digestion. 2003;68:184-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Warrington S, Baisley K, Boyce M, Tejura B, Morocutti A, Miller N. Effects of rabeprazole, 20 mg, or esomeprazole, 20 mg, on 24-h intragastric pH and serum gastrin in healthy subjects. Aliment Pharmacol Ther. 2002;16:1301-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Baisley K, Warrington S, Tejura B, Morocutti A, Miller N. Rabeprazole 20 mg compared with esomeprazole 40 mg in the control of intragastric pH in healthy volunteers. Gut. 2002;50:A63. [Cited in This Article: ] |
| 20. | Pantoflickova D, Dorta G, Ravic M, Jornod P, Blum AL. Acid inhibition on the first day of dosing: comparison of four proton pump inhibitors. Aliment Pharmacol Ther. 2003;17:1507-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalväg A. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Galmiche JP, Barthelemy P, Hamelin B. Treating the symptoms of gastro-oesophageal reflux disease: a double-blind comparison of omeprazole and cisapride. Aliment Pharmacol Ther. 1997;11:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol. 1997;32:965-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 200] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Fletcher J, Wirz A, Young J, Vallance R, McColl KE. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology. 2001;121:775-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Bytzer P, Blum A, De Herdt D, Dubois D. Six-month trial of on-demand rabeprazole 10 mg maintains symptom relief in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2004;20:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |