INTRODUCTION
Focal adhesion kinase (FAK) is a cytoplasmic protein tyrosine kinase that has been implicated to play an important role in integrin-mediated signal transduction pathways. Furthermore, some lines of evidence indicate FAK as a point of convergence of other signaling pathways. The in vitro expression of FAK and its level of phosphorylation appear to be related to several physiological phenomena, including cell adhesion, spreading, migration, cytoskeleton organization, proliferation and apoptosis[1-3]. It has been accepted that hepatic stellate cells (HSCs) represent the pivot of fibrotic process[4-9]. In healthy livers, HSCs are perisinusoidal mesenchymal elements with characteristic intracytoplasmatic lipid droplets rich in retinyl esters. In contrast, in chronic liver injury, HSCs undergo a process of activation from the resting fat-storing phenotype towards a myofibroblast-like phenotype[10].
Current evidence indicates that activated HSCs are responsible for the majority of extracellular matrix protein depositions in liver fibrosis[11]. Although a recent study has focused on the roles of FAK in cultured cells[12], little is known about its regulation in vivo or its relevance to HSC proliferation. In addition, it is not known whether FAK is up-regulated in response to liver injury by bile duct ligation (BDL). To assess whether FAK was associated with fibrogenesis or the survival of HSCs in biliary fibrosis, we investigated the expression of FAK in liver tissues from rats with BDL-induced biliary fibrosis using immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR), we also used immunohistochemistry method to examine the expression of α -smooth muscle actin (α -SMA) as a marker of activated HSCs.
MATERIALS AND METHODS
Reagents
Monoclonal antibodies against FAK and α -SMA were products of Santa Cruz Biotech Inc. Streptavidin peroxidase (SP) immunohistochemical kit was purchased from Zhongshan Biological Technology Co. (Beijing). Trizol reagent was obtained from Life Technologies, Inc (USA). One tube RT-PCR kit was from Promega Co (USA). Primers for rat FAK and β -actin were designed by ourselves in accordance with gene sequence in GeneBank, synthesized and purified by Bao Biological Engineering Co. (Dalian). All other reagents were analytically pure.
Animal model and experimental protocol
A total of 80 adult male Sprague-Dawley rats weighing 350-400 g were purchased from the Experimental Animal Center of Hebei Medical University (Clearing Grade, Certificate No. 04057). All rats were housed in plastic cages and allowed free access to food and water. For the purpose of this study, rats were randomly divided into eight groups (ten rats in each group) as follows: Control group (sham-operated group), with BDL for 2 h, 6 h, 2 d, 1 wk, 2 wk, 3 wk and 4 wk, respectively. The rats were subjected to laparotomy with their common bile ducts completely ligated while they were intraperitoneally injected with ketamine hydrochloride at a dose of 100 mg/kg[13]. Under deep anaesthesia, the peritoneal cavity was opened and the common bile duct was double-ligated with 3-0 silk and cut between the ligatures. Control animals underwent a sham operation that consisted of exposure but not ligation of the common bile duct. At various intervals postoperation, animals were anaesthetised and the livers were harvested. Liver tissue specimens were routinely fixed in 40 g/L phosphate-buffered formaldehyde and embedded in paraffin. Some liver tissue specimens were used for light microscopy and immunohistochemistry using anti α -SMA and FAK, while others were snap-frozen in liquid nitrogen and stored at -80 °C for RNA analysis. In addition, control livers were harvested 4 wk after sham operation.
Histopathology
For light microscopic examination, liver specimens were routinely fixed overnight in 40 g/L phosphate-buffered formaldehyde, embedded in paraffin. Tissue sections (5-μm thick) were stained with haematoxylin and eosin (HE) for morphological evaluation and Masson’s trichrome for assessment of fibrosis.
Immunohistochemical detection of α -SMA and FAK
All immunohistochemical studies using the streptavidin-peroxidase technique were performed on 5-µm thick sections of paraformaldehyde-fixed and paraffin-embedded liver block tissue mounted on APES-coated slides. Slides were deparaffinised in xylene, and rehydrated in graded ethanol. Endogenous peroxidase activity was quenched with a 30 mL/L hydrogen peroxide solution in methanol at room temperature for 30 min, followed by rinsing in pH 6.0 phosphate-buffered saline (PBS). After antigen retrieval in a water bath set in a 10 mmol/L citrate buffer (pH 6.0) at 94 °C for 8 min, 10 min, respectively, the slides were immediately cooled for 20 min at room temperature. Non-specific binding sites were blocked by incubation with washing buffer containing 100 mL/L normal goat serum at 37 °C for 30 min. Sectionsrnight at 4 °C with a mouse monoclonal antibody directed against α -SMA or FAK at a dilution of 1:100. The secondary antibody bindings were localized using a biotin conjugated rabbit anti-mouse IgG (1:100 dilution), followed by incubation with streptavidin-peroxidase complex (1:200 dilution). Peroxidase conjugates were subsequently visualised using diaminobenzidine (DAB) solution in hydrogen peroxide as a chromogen yielding a brown reaction product. Sections were then counterstained in Mayer’s hematoxylin and mounted over cover slips. All incubations were performed in a moist chamber. Furthermore, between each incubation step, the slides were washed three times with PBS for 5 min. To ensure antibody specificity, negative control samples were processed in parallel under the same conditions but with omission of the first antibody, which was replaced by an equal volume of PBS. The α -SMA-positive parenchyma and the FAK-positive parenchyma were measured by a video-image analysis system and expressed as a percentage of area occupied by the signal.
RNA extraction and RT -PCR assay
Expression of FAK mRNA was evaluated with RT-PCR. Total RNA from liver specimens (100 mg) was isolated using a monophasic solution of phenol and guanidine thiocyanate (Trizol), precipitated in ethanol and resuspended in sterile RNAase-free water for storage at -80 °C until use, as recommended by the suppliers. Total RNA was quantified spectrometrically at 260 nm, and the quality of isolated RNA was analysed on agarose gels under standard conditions. One-step RT-PCR was performed according to the manufacturer’s instructions. Two micrograms of RNA was added to each reaction and RT-PCR was routinely performed using 5 units of AMV reverse transcriptase, 5 units of Tfl DNA polymerase, 10 pmoL of each oligonucleotide primer, 10 pmoL of dNTP mix and 25 mmol/L MgSO4 in a final reaction volume of 50 µL. Primer sequences were as follows: FAK, forward 5’-ACT TGG ACG CTG TAT TGG AG-3’ and reverse 5’-CTG TTG CCT GCT TTC TGG AT-3’, fragment length 833 bp; β -actin, forward 5’- AGC TGA GAG GGA AAT CGT GCG -3’ and reverse 5’- GTG CCA CCA GAC AGC ACT GTG -3’, fragment length 300 bp. RT-PCR was performed in the following steps: reverse transcription was performed at 41 °C for 45 min, pre-denaturation at 94 °C for 2 min; then amplification was performed in a thermal controller for 35 cycles (denaturation at 94 °C for 40 s, annealing at 52 °C for 1 min and extension at 72 °C for 1.5 min), and a final extension at 72 °C for 10 min after the last cycle. A 10 µL of the PCR products was analyzed on 15 g/L agarose gel containing ethidium bromide with TAE buffer at 80 V for 40 min and photographed under UV illumination. The band intensities were quantified by densitometry. FAK/β -actin quotient indicated the relative expression of FAK. Experiments were performed at least three times with similar results.
Statistical analysis
The data were expressed as mean ± SD. The mean values were compared by using analysis of variance, followed by the Student-Newman-Keuls test if the former was significant. The correlation between the expressions of FAK and α -SMA was analyzed for statistical significance by the simple linear regression analysis. P values less than 0.05 were considered statistically significant.
RESULTS
Histology of progressive fibrotic liver injury
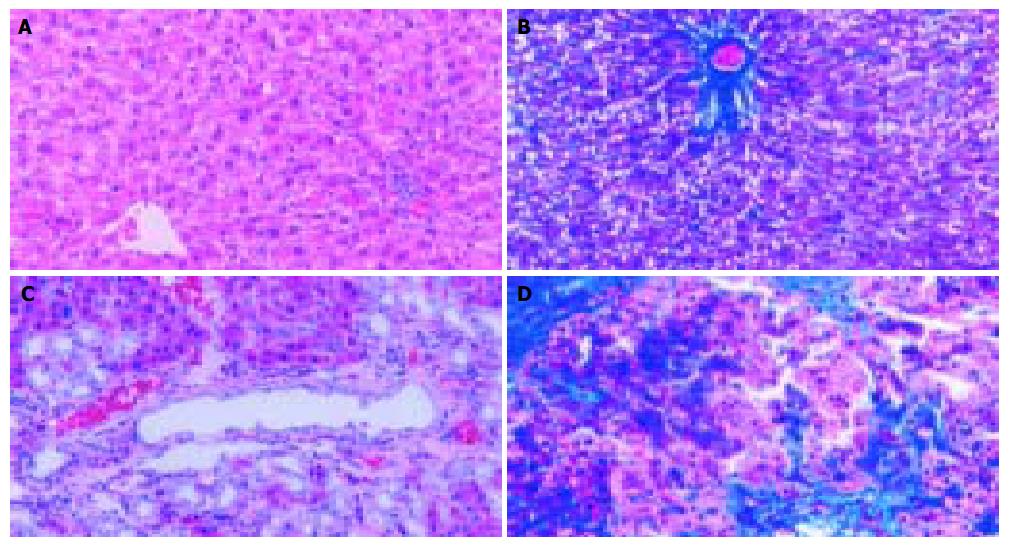
In accordance with previous reports[13], a marked liver fibrosis was apparently observed in the SD rats after BDL. In the present study, the results of HE and Masson’s trichrome staining confirmed spotted (or scattered) perivenular degeneration of hepatocytes, an increase in the inflammatory infiltrates in the necrotic areas and bile ductular proliferation in the portal triads after 1 wk of BDL. After 2 wk of BDL, all rats showed expanded portal tracts with fibrous tissues, portal-to-portal fibrous bridging, nodular transformation and widespread proliferating bile ductules that extended into the parenchyma in places without clear-cut cirrhosis. After BDL for 3-4 wk, the animals developed severe fibrosis associated with proliferating bile ducts that formed a continuous meshwork of connective tissues with complete distortion of lobular architecture, whereas there was no notable histological abnormality or evidence of stainable collagen in any of the shamly operated control livers (Figure 1A-D).
Figure 1 Histopathological changes in liver tissue (100 × ) A: Normal hepatic lobular architecture in sham operation group (HE); B: Few ECM deposition in sham operation group (Massson trichome); C: Extensive ductular proliferation and ECM deposition 4 wk after BDL (HE); D: Extensive connective tissue deposition 4 wk after BDL (Massson trichome).
Identification of proliferating and activated HSC
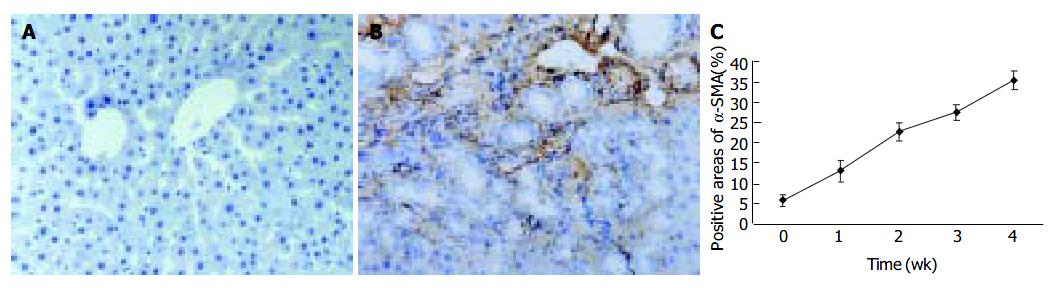
Since α -SMA was expressed in activated HSCs, immunostaining for this protein was used to detect and quantify the numbers of activated HSCs. A weak staining for α -SMA positive cells in the shamly operated control livers was observed in vascular smooth muscle cells and sinusoids. With the development of hepatic fibrosis, the positive stainings for α -SMA were greatly increased in the cells of portal ducts, fibrotic septa, perisinuses and around the proliferated bile ducts. The positively stained areas of the rat livers in model groups 1 to 4 wk after ligation of common bile duct (12.88 ± 2.63%, 22.65 ± 2.16%, 27.45 ± 1.86%, 35.25 ± 2.34%, respectively) were significantly larger than that in control group (5.88 ± 1.46%) ( P < 0.01, Figure 2 A-C).
Figure 2 α -SMA protein expression in liver tissue stained by immunohistochemistry (SP 200 × ) A: Few α -SMA expressions in sham operation group; B: Positive cells of α -SMA resided in portal ducts, fiber septa, perisinuses and around proliferated bile ducts after 2 wk BDL; C: Positively stained areas of α -SMA expression in model groups 1 to 4 wk after common bile duct ligation.
Distribution of FAK protein in common bile duct ligated rat liver
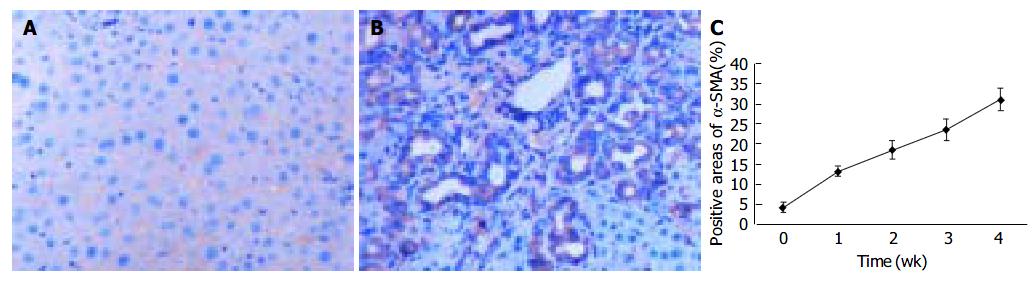
To explore the distribution of FAK, the sections of rat livers in sham operation and BDL groups were immunostained using specific monoclonal anti-FAK antibody. In the sections from sham operation group, expression of FAK was found in vascular smooth muscle cells and perisinusoidal cells. In contrast, the positive staining of BDL rat liver was obviously visible in and around α -SMA-positive areas, which were prevailingly detected in portal ducts, fibrotic septa, perisinusoidal cells and cells around the bile ducts as well as vascular endothelial cells. The positive areas of the rat livers in model groups 1 to 4 wk after ligation of common bile duct (13.05 ± 1.32%, 18.43 ± 2.18%, 23.45 ± 2.73%, 31.00 ± 2.77%, respectively) were significantly larger than that in control group (3.98 ± 1.27%) ( P < 0.01, Figure 3A-C).
Figure 3 FAK protein expression in liver tissue stained by immunohistochemistry (SP 200 × ) A: FAK protein expression in sham operation group; B: FAK protein expression 2 wk after BDL; C: Time course of FAK expression in hepatic fibrogenesis stained by immunohistochemistry.
FAK mRNA expression in common bile duct ligated rat livers
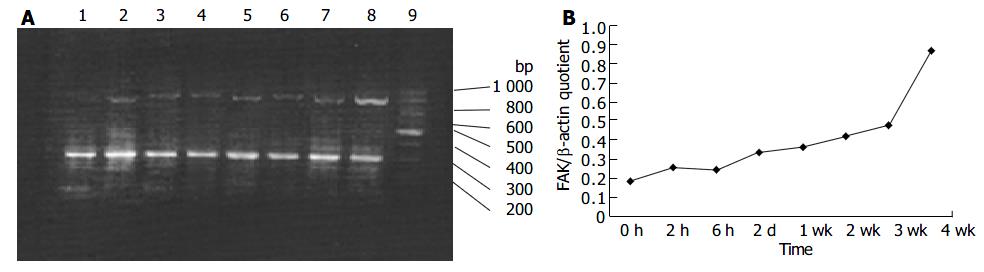
Although it was shown that FAK protein was produced by liver tissues in vivo, it was not clear whether the FAK mRNA level under fibrogenic response was increased in vivo. Therefore, we investigated the production of FAK mRNA in the liver. RT-PCR results revealed faint bands for FAK mRNA in the sham operation group, whereas obvious and specific bands for FAK mRNA in fibrotic liver of BDL groups. Moreover, FAK mRNA expression was initially up-regulated and reached the peak level 4 wk after BDL. The levels housekeeping gene, β -actin, did not show any significant differences between normal and BDL rat liver tissues (Figure 4A, B).
Figure 4 RT-PCR analysis of mRNA encoding FAK in hepatic fibrogenesis A: RT-PCR analysis of mRNA encoding FAK in hepatic fibrogenesis at different time points.
Lane 1: sham operation group; lane 2: BDL 2 h; lane 3: BDL 6 h; lane 4: BDL 2 d; lane 5: BDL 1 wk; lane 6: BDL 2 wk; lane 7: BDL 3 wk; lane 8: BDL 4 wk; lane 9: Marker B: Band intensities were quantified by densitometry. FAK/β -actin quotient indicated the relative expression of FAK.
Correlation between FAK and α -SMA
Immunohistochemistry experiments were performed to analyze whether FAK protein distribution was correlated with α -SMA between sham operation group and BDL group. The results indicated that FAK was positively correlated with α -SMA (r =0.963, P < 0.05).
DISCUSSION
HSCs, a principal cellular source of extracellular matrix during chronic liver injury, undergo a transition into α -SMA-expressing myofibroblast-like cells in response to injury. Furthermore, HSC activation is associated with stellate cell proliferation, increased contractility, enhanced matrix production, and expression of a number of fibrogenic and proliferative cytokines and their cognate receptors. Therefore, HSCs could play a pivotal role in cellular and molecular events that lead to fibrosis[14-17]. As above, expressing α -SMA is one of the characteristics of activated HSCs. Cassiman et al[18] and α - SMA-positive cells mainly reside in the portal triads and fibrotic septa accompanied with proliferating bile ducts. Namely,α - SMA-positive cells was coincident with collagen deposition. The results of the present study verified that the positive cells of α -SMA, which mainly reside in portal ducts, fibrotic septa, perisinuses and around the proliferated bile ducts, were greatly increased with the development of hepatic fibrosis compared with sham operation group. Thus, our results are consistent with the others mentioned above.
Various factors and signal transduction pathways have been shown to regulate the activation and proliferation of HSCs[8,9,19-21]. However, the mechanism by which in vivo factors impact HSC activation is not clear as yet. To better understand the mechanism by which FAK is generated in liver tissues might influence HSC activation, proliferation, and hepatic fibrogenesis, we performed the current research.
FAK, a 125 kD molecule, is a cytoplasmic nonreceptor tyrosine kinase that has been shown to play a key role in the regulation of cell adhesion, spreading, migration, cytoskeleton organization, proliferation and apoptosis. Furthermore, some lines of evidence has indicated FAK is a point of convergence of many signaling pathways[22]. The co-localization of FAK with integrins in focal adhesion plaque (FAP) is a trigger for cell adhesion-dependent activation of FAK signals[22] . Subsequently, its autophosphorylation at Tyr397 in the N-terminal domain is prerequisite, which may initiate a number of signaling pathways. Among them, RAS-dependent mitogen-activated protein kinase (MAPK) pathway is the clearest. It has been found that the RAS-RAF-MEK (ERK kinase)-ERK (extracellular signal-regulated kinase) pathway is involved in many cellular behaviors[22,23], such as proliferation, apoptosis. FAK knockout mice showed extensive mesodermal defects and embryonic death[24]. Moreover, the monoclonal antibody specific for FAK microinjected into fibroblasts could inhibit FAK activity and give rise to apoptosis of fibroblasts[25]. The results presented herein demonstrated that FAK protein was obviously expressed in portal ducts, fibrotic septa, perisinusoidal cells in BDL rat livers, which was in accordance was the distribution α -SMA-positive loci. In addition, FAK mRNA expression was also elevated with the progression of hepatic fibrosis. Importantly, FAK protein distribution was positively correlated with α -SMA. So, we suggested that activation of FAK in BDL rat liver tissues might activate downstream signal molecules, which could modulate gene expression of HSCs and give rise to hepatic fibrosis.
To date, the mechanism of FAK elevation during hepatic fibrogenesis remains unknown. But increasing in vitro evidence supports that HSCs are a major cell source of FAK. First, extracellular matrix components, including collagen and fibronectin could activate FAK in HSCs via integrin signal pathway[26-28]. Second, cytokines such as platelet derived growth factor (PDGF), endothelin (ET), insulin-like growth factor-1 (IGF-1) and tumor necrosis factor (TNF), could increase the production of FAK[29]. Third, reactive oxygen species (ROS) like H2O2 could also elevate the expression of FAK[30,31]. In contrast, antioxidants such as Salvia Miltiorrhiza, might have opposite effects on FAK expression in HSCs[32,33].
In conclusion, FAK-mediated activation of HSCs can result in hepatic fibrogenesis. Inhibition of FAK activity by various methods would be expected to attenuate liver fibrosis and, therefore, deserve further study.