Copyright
©The Author(s) 2024.
World J Gastroenterol. Mar 7, 2024; 30(9): 1237-1249
Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1237
Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1237
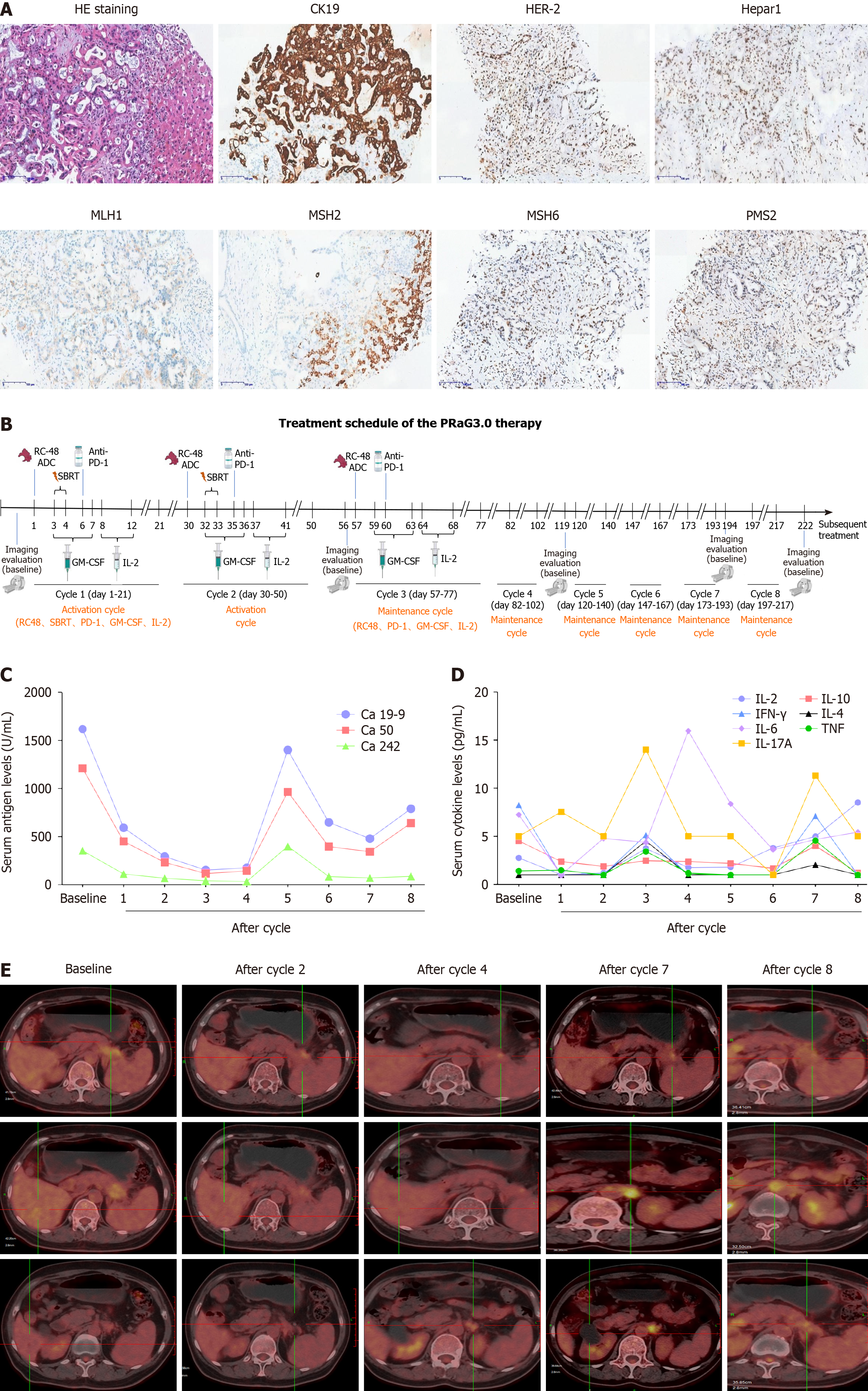
Figure 1 Treatment schedule of the PRaG3.
0 therapy and treatment efficacy evaluation of the patient. A: Hematoxylin and eosin staining and immunohistochemistry assay for CK19, human epidermal growth factor receptor 2 (HER-2), Hepar1, MLH1, MSH2, MSH6 and PMS2 of liver lesions. CK19 was expressed in tumor cells but Hepar1 was not expressed. HER-2 was weakly expressed in tumor cell membranes. MLH1, MSH2, MSH6 and PMS2 were expressed in tumors indicating mismatch repair proficient; B: Treatment schedule of the PRaG3.0 therapy. The patient received two activation cycle and six maintenance cycle. The time of positron emission tomography-computed tomography (PET-CT) was also indicated; C: Serum carbohydrate antigen 19-9 (Ca 19-9), Ca 50 and Ca 242 levels of the patient during the treatment; D: Peripheral cytokines [interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor and interferon-γ] levels changes of the patient during the treatment. E: The PET-CT evaluation of the patient at baseline and after cycle 2, 4, 7 and 8. ADC: Antibody-drug conjugate; Ca: Carbohydrate antigen; GM-CSF: Granulocyte-macrophage colony-stimulating factor; HE: Hematoxylin and eosin; HER-2: Human epidermal growth factor receptor 2; IFN: Interferon; IL: Interleukin; SBRT: Stereotactic body radiotherapy; TNF: Tumor necrosis factor.
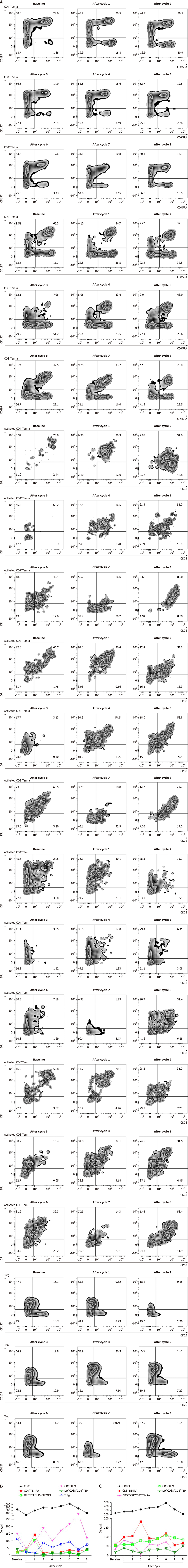
Figure 2 The percentage and absolute numbers of lymphocyte subsets at baseline and after each cycle.
A: The percentage of peripheral lymphocytes, including CD4+Temra, CD8+Temra, activated CD4+Temra, activated CD8+Temra, activated CD4+Tem, activated CD8+Tem and Tregs at baseline and after each cycle; B and C: Absolute numbers of peripheral lymphocytes subsets at baseline and after each cycle.
- Citation: Kong YH, Xu ML, Zhang JJ, Chen GQ, Hong ZH, Zhang H, Dai XX, Ma YF, Zhao XR, Zhang CY, Chen RZ, Xing PF, Zhang LY. PRaG 3.0 therapy for human epidermal growth factor receptor 2-positive metastatic pancreatic ductal adenocarcinoma: A case report. World J Gastroenterol 2024; 30(9): 1237-1249
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1237