Copyright
©The Author(s) 2023.
World J Gastroenterol. Mar 7, 2023; 29(9): 1395-1426
Published online Mar 7, 2023. doi: 10.3748/wjg.v29.i9.1395
Published online Mar 7, 2023. doi: 10.3748/wjg.v29.i9.1395
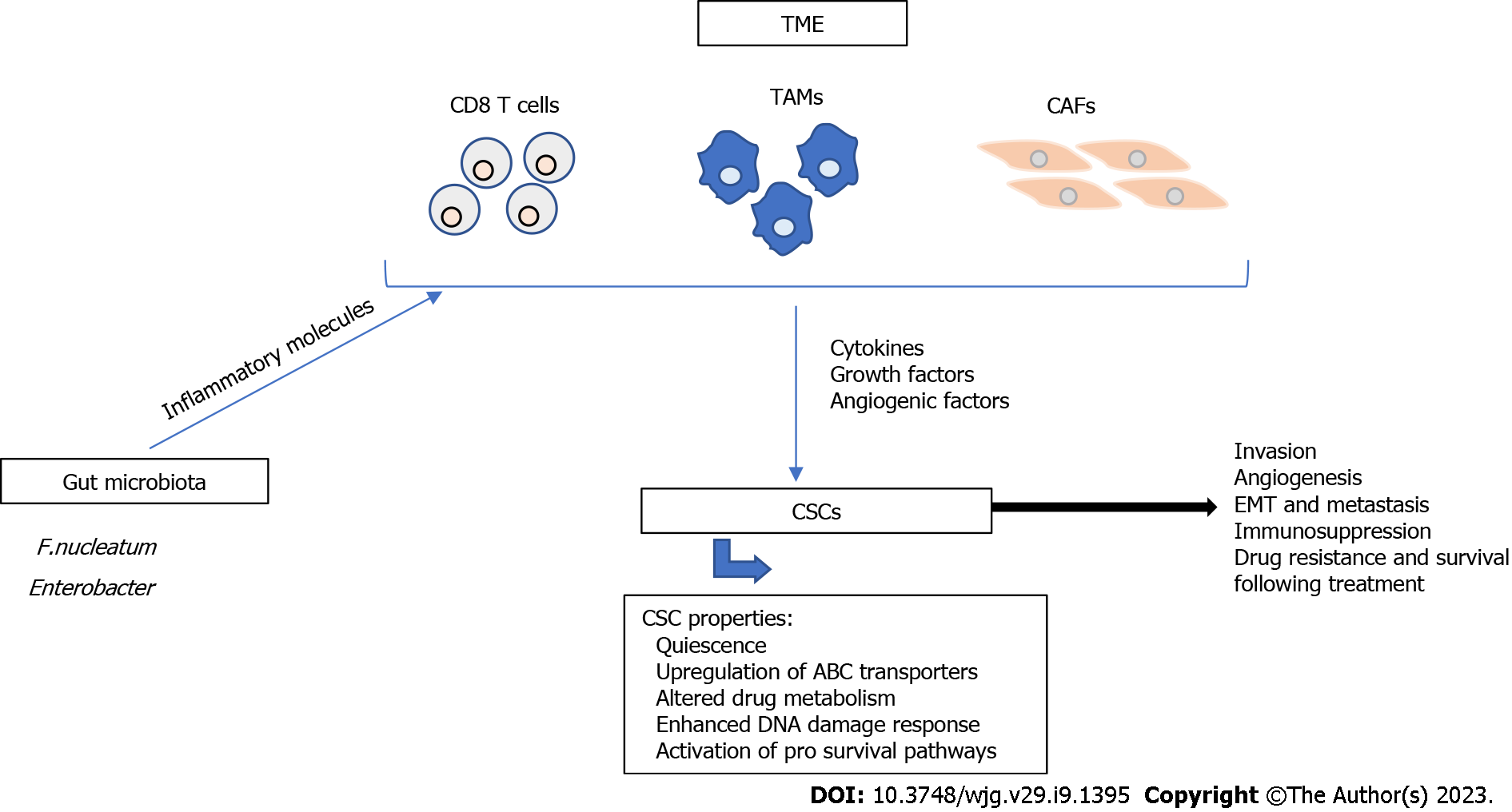
Figure 1 Major mechanisms of cancer stem cell resistance.
Cancer stem cell (CSC) resistance has been associated with CSC characteristics including quiescence, upregulation of ATP-binding cassette transporters, altered drug metabolism, enhanced DNA damage response, and activation of pro survival pathways. The tumor microenvironment (TME) plays a major role in the resistance of CSCs to therapy. CD8 T cells, tumor associated macrophages, and cancer associated fibroblasts (CAFs) are major components of the TME and contribute to tumor progression and metastasis through the secretion of cytokines, growth factors, and angiogenic factors. Additionally, gut microbiota, such as Fusobacterium nucleatum and Enterobacter secrete inflammatory molecules that modulate the TME and contribute to therapy resistance. All of these mechanisms contribute to tumor invasion, angiogenesis, epithelial-to-mesenchymal transition, immunosuppression, drug resistance and survival following treatment. TAMs: Tumor associated macrophages; CAFs: Cancer associated fibroblasts; EMT: Epithelial-to-mesenchymal transition.
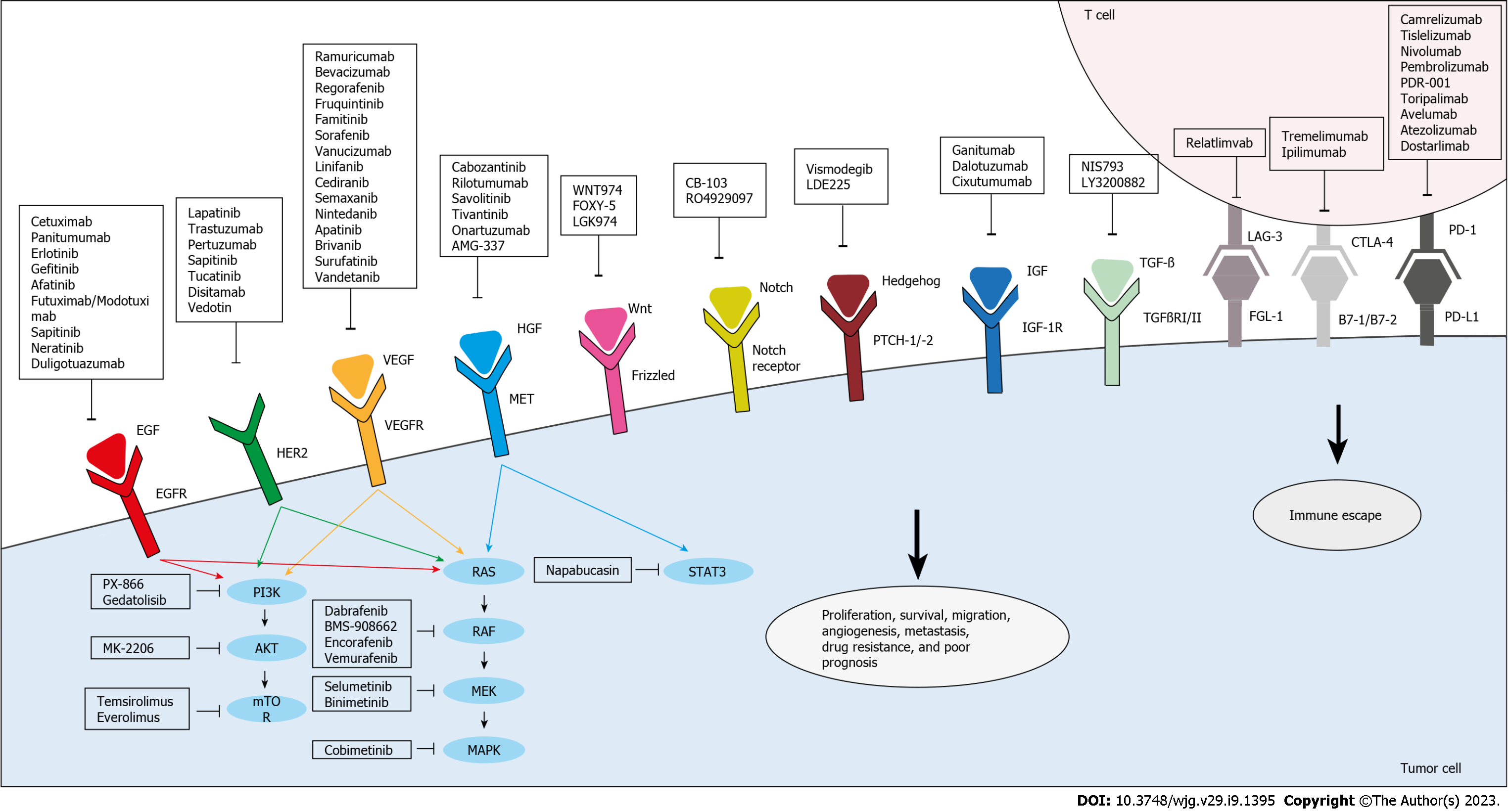
Figure 2 Targeted therapies under investigation for the treatment of drug-resistant and metastatic colorectal cancer.
Anti-epidermal growth factor receptor, anti-vascular endothelial growth factor/vascular endothelial growth factor receptor, and anti-human epidermal growth factor receptor 2 agents inhibit their respective targets and thus, the downstream effector pathways, PI3K/Akt and RAS/RAF. Other agents directly target and inhibit PI3K, AKT, mammalian target of rapamycin, RAF, mitogen-activated extracellular signal-regulated kinase, or mitogen-activated protein kinases. In addition, anti-hepatocyte growth factor/mesenchymal epithelial transition factor receptor agents target this pathway to inhibit signal transducer and activator of transcription, which is also targeted by Napabucasin. Several novel agents that are aimed at other pathways implicated in colorectal cancer proliferation, survival, resistance, and metastasis are also being evaluated. Targeted pathways include Wnt, Notch, Hedgehog, insulin growth factor/insulin growth factor receptor-1, and transforming growth factor beta. Moreover, immune escape can be hindered through immunotherapy which targets co-inhibitory molecules, mainly programmed death-1/programmed death ligand-1, cytotoxic T lymphocyte-associated antigen 4, and lymphocyte activation gene 3. EFGR: Epidermal growth factor receptor; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; HER2: Human epidermal growth factor receptor 2; mTOR: Mammalian target of rapamycin; MEK: Mitogen-activated extracellular signal-regulated kinase; MAPK: Mitogen-activated protein kinases; HGF: Hepatocyte growth factor; MET: Mesenchymal epithelial transition factor receptor; STAT3: Signal transducer and activator of transcription; CRC: Colorectal cancer; IGF: Insulin growth factor; IGF-1R: Insulin growth factor receptor-1; TGF-β: Transforming growth factor beta; PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; CTLA-4: Cytotoxic T lymphocyte-associated antigen 4; LAG-3: Lymphocyte activation gene 3; EGF: Epidermal growth factor; TGFβRI/II: Transforming growth factor-Beta type I/II.
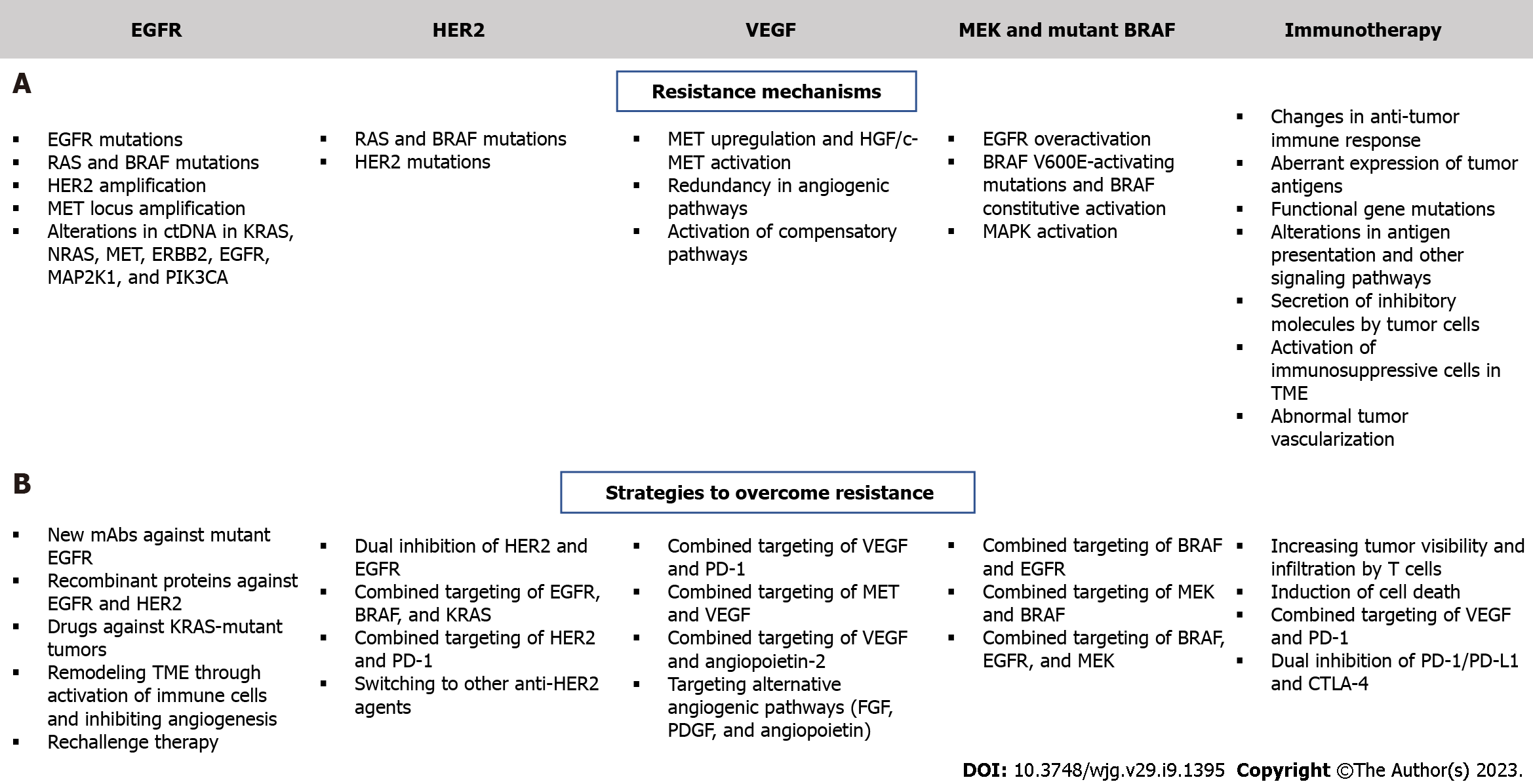
Figure 3 Mechanisms of resistance to targeted therapy and strategies to overcome resistance in colorectal cancer.
A: Resistance mechanisms; B: Strategies to overcome resistance. EGFR: Epidermal growth factor receptor; HER2: Human epidermal growth factor receptor 2; VEGF: Vascular endothelial growth factor; MEK: Mitogen-activated extracellular signal-regulated kinase; MAP2K1: Mitogen-Activated Protein Kinase 1; PI3KCA: Phosphoinositide 3-kinases catalytic subunit alpha; MAP2K1: Mitogen-activated protein kinase 1; HGF: Hepatocyte growth factor; MET: Mesenchymal epithelial transition; ERBB2: Erb-B2 Receptor Tyrosine Kinase 2; TME: Tumor microenvironment; FGF: Fibroblast growth factor; PDGF: Platelet-derived growth factor; PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; CTLA-4: cytotoxic T lymphocyte-associated antigen 4.
- Citation: Al Bitar S, El-Sabban M, Doughan S, Abou-Kheir W. Molecular mechanisms targeting drug-resistance and metastasis in colorectal cancer: Updates and beyond. World J Gastroenterol 2023; 29(9): 1395-1426
- URL: https://www.wjgnet.com/1007-9327/full/v29/i9/1395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i9.1395