Copyright
©The Author(s) 2023.
World J Gastroenterol. Jul 28, 2023; 29(28): 4451-4465
Published online Jul 28, 2023. doi: 10.3748/wjg.v29.i28.4451
Published online Jul 28, 2023. doi: 10.3748/wjg.v29.i28.4451
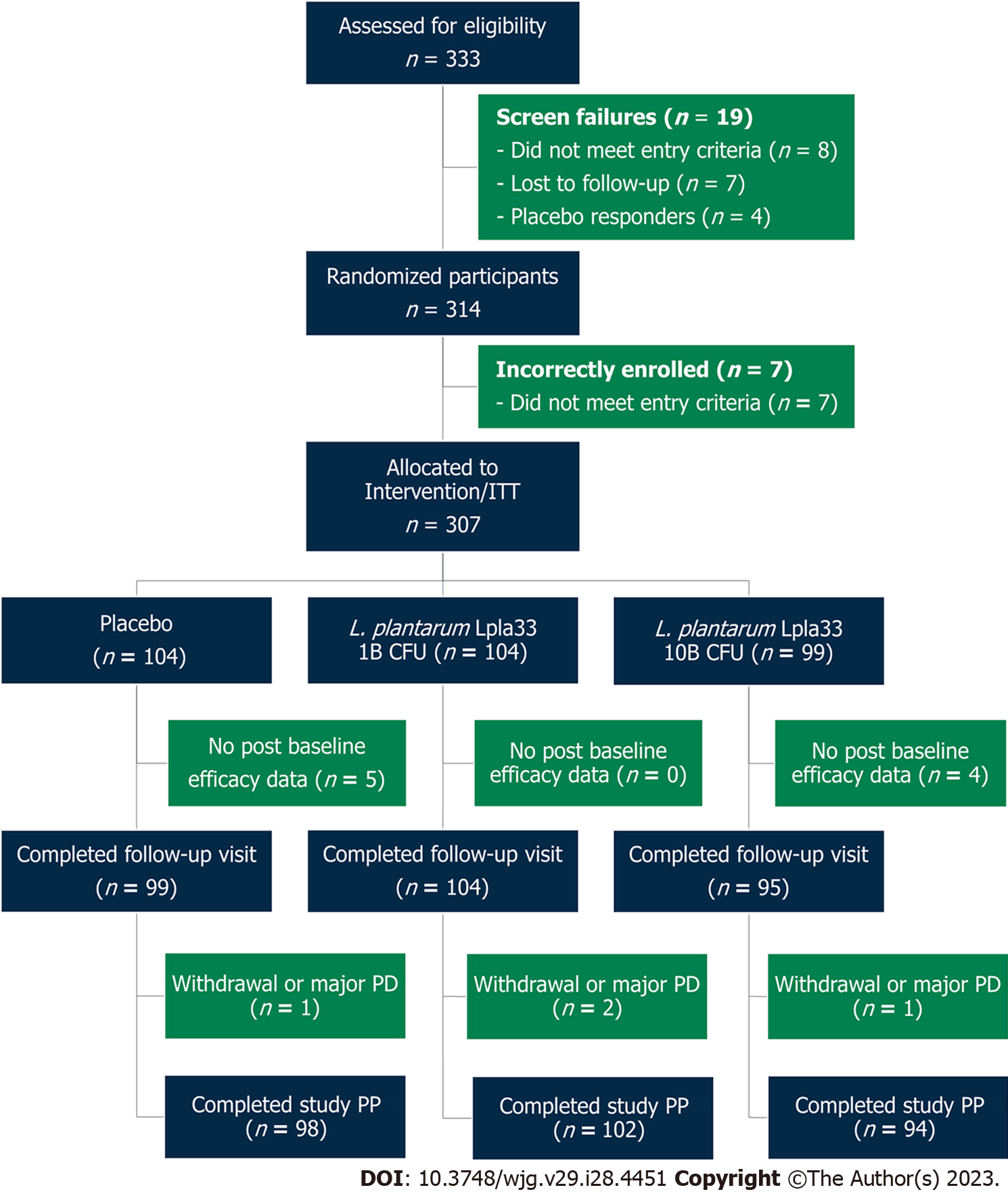
Figure 1 Participant flow chart.
ITT: Intention-to-treat; PD: Protocol deviation; PP: Per protocol; L. plantarum: Lactiplantibacillus plantarum.
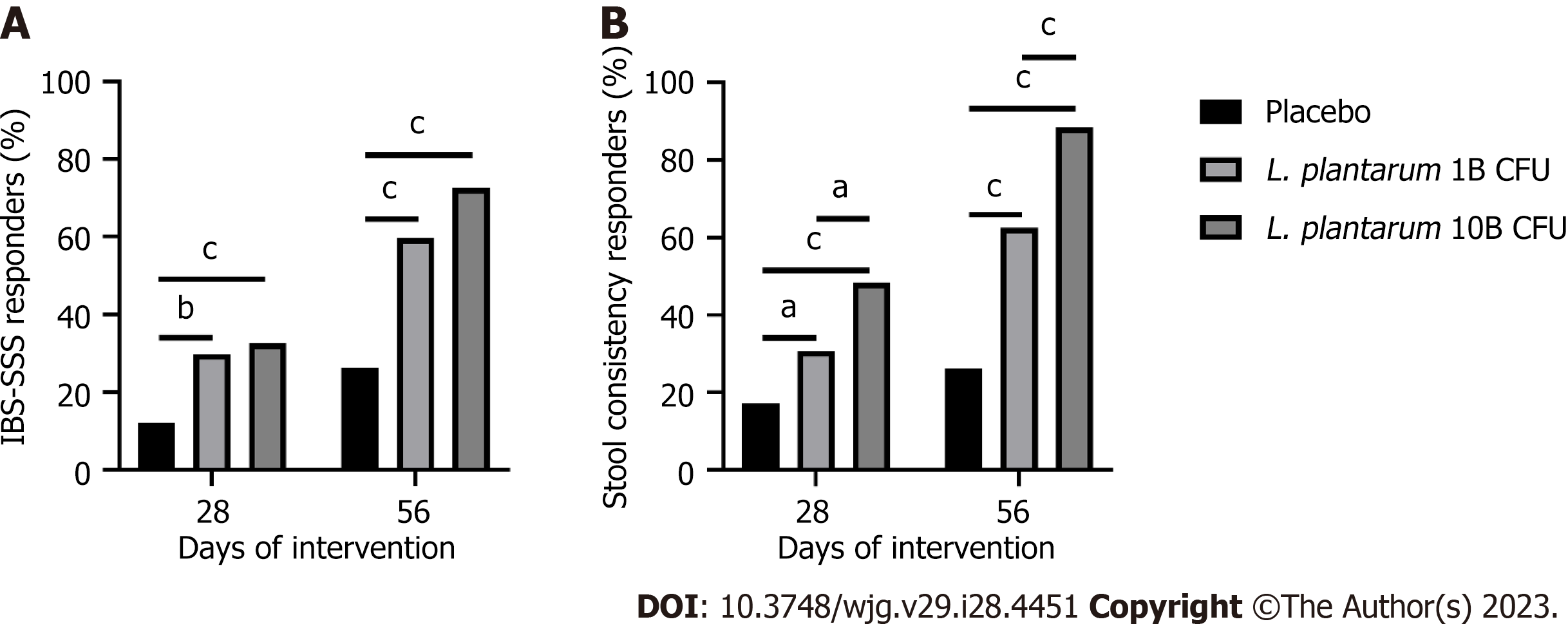
Figure 2 Responder profile over the intervention period.
A: Percentage of irritable bowel syndrome-severity scoring system (IBS-SSS) responders, as defined by a decrease of 95 points or more in the IBS-SSS total score from baseline, in participants receiving placebo or Lactiplantibacillus plantarum (L. plantarum) (1B or 10B CFU) capsules; B: Percentage of stool consistency responders, as defined by a decrease of at least 50% in the number of days per week with at least one stool that has a consistency of type 6 or 7 compared with baseline, in participants receiving placebo or L. plantarum (1B or 10B CFU) capsules. Between group comparison via Pearson chi-square test. aP < 0.05; bP < 0.01; cP < 0.001. L. plantarum: Lactiplantibacillus plantarum.
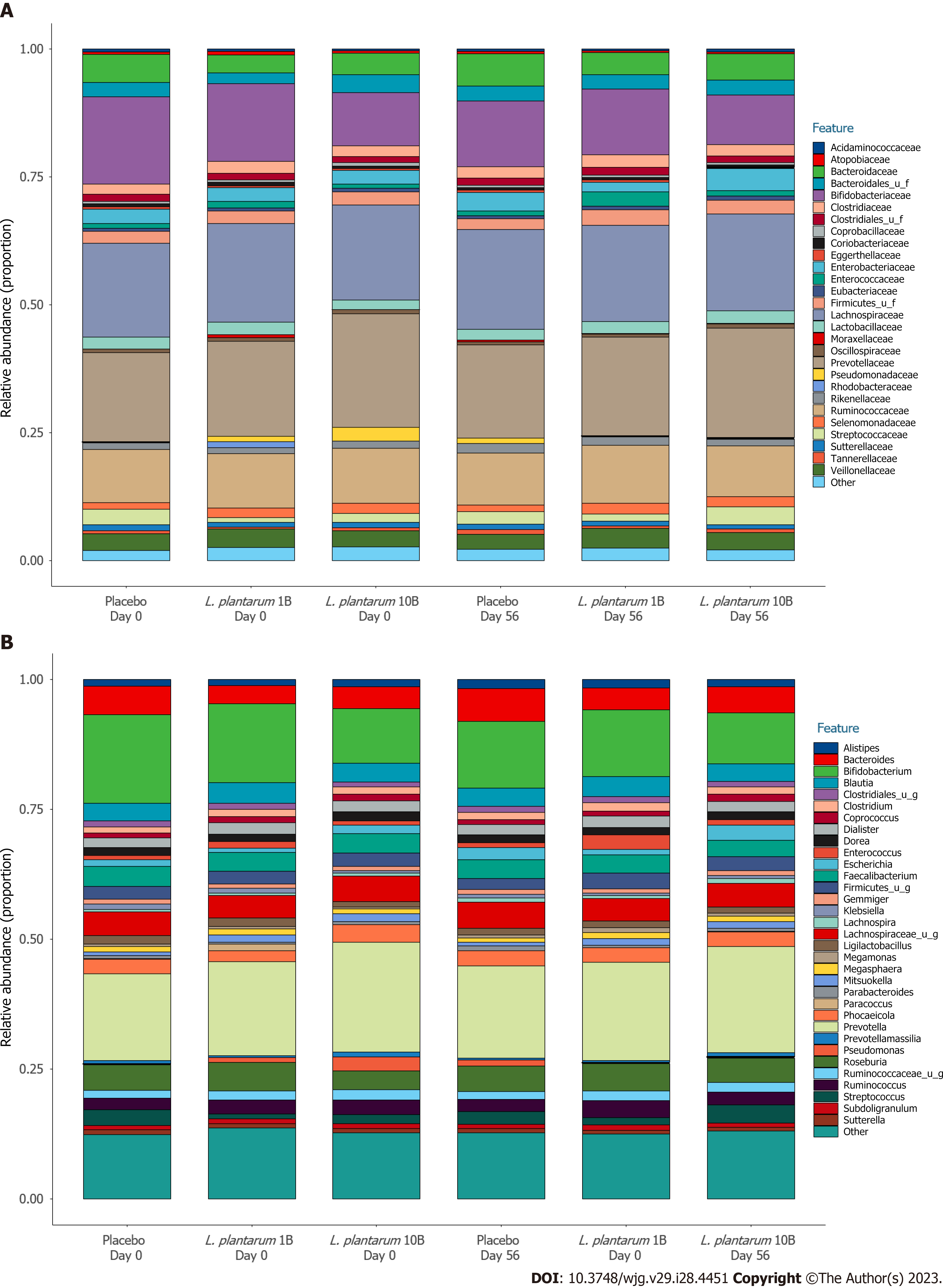
Figure 3 Fecal microbial profile over the intervention period.
A: Proportional family level abundance in participants receiving placebo or Lactiplantibacillus plantarum (L. plantarum) (1B or 10B CFU) capsules; B: Proportional genus level abundance in participants receiving placebo or L. plantarum (1B or 10B CFU) capsules.
- Citation: Martoni CJ, Srivastava S, Damholt A, Leyer GJ. Efficacy and dose response of Lactiplantibacillus plantarum in diarrhea-predominant irritable bowel syndrome. World J Gastroenterol 2023; 29(28): 4451-4465
- URL: https://www.wjgnet.com/1007-9327/full/v29/i28/4451.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i28.4451