Copyright
©The Author(s) 2022.
World J Gastroenterol. Nov 28, 2022; 28(44): 6294-6309
Published online Nov 28, 2022. doi: 10.3748/wjg.v28.i44.6294
Published online Nov 28, 2022. doi: 10.3748/wjg.v28.i44.6294
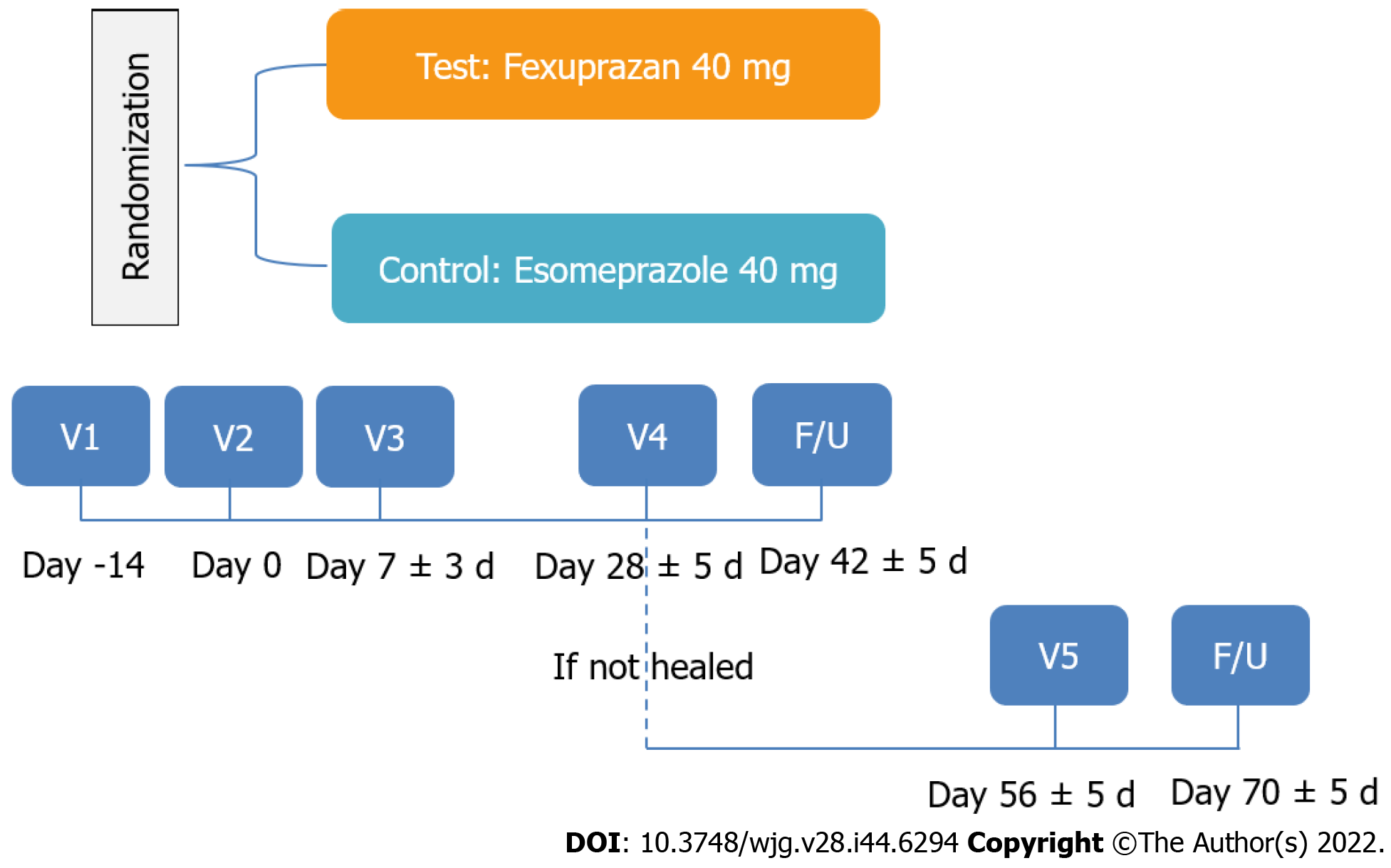
Figure 1 Study schema.
V: Visit; F/U: Follow-up.
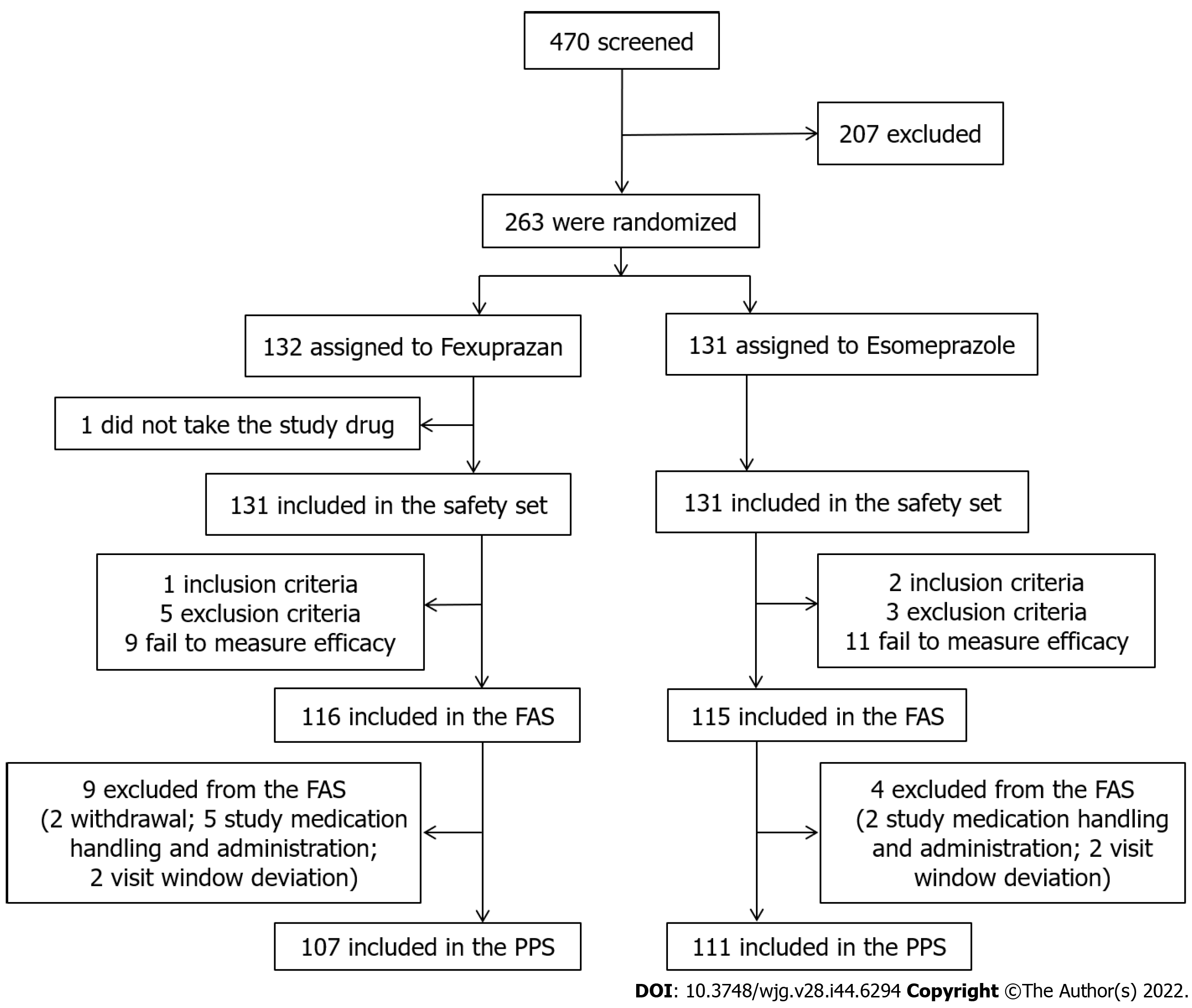
Figure 2 Flowchart of the study patients.
SS: Safety set; FAS: Full analysis set; PPS: Per-protocol set.
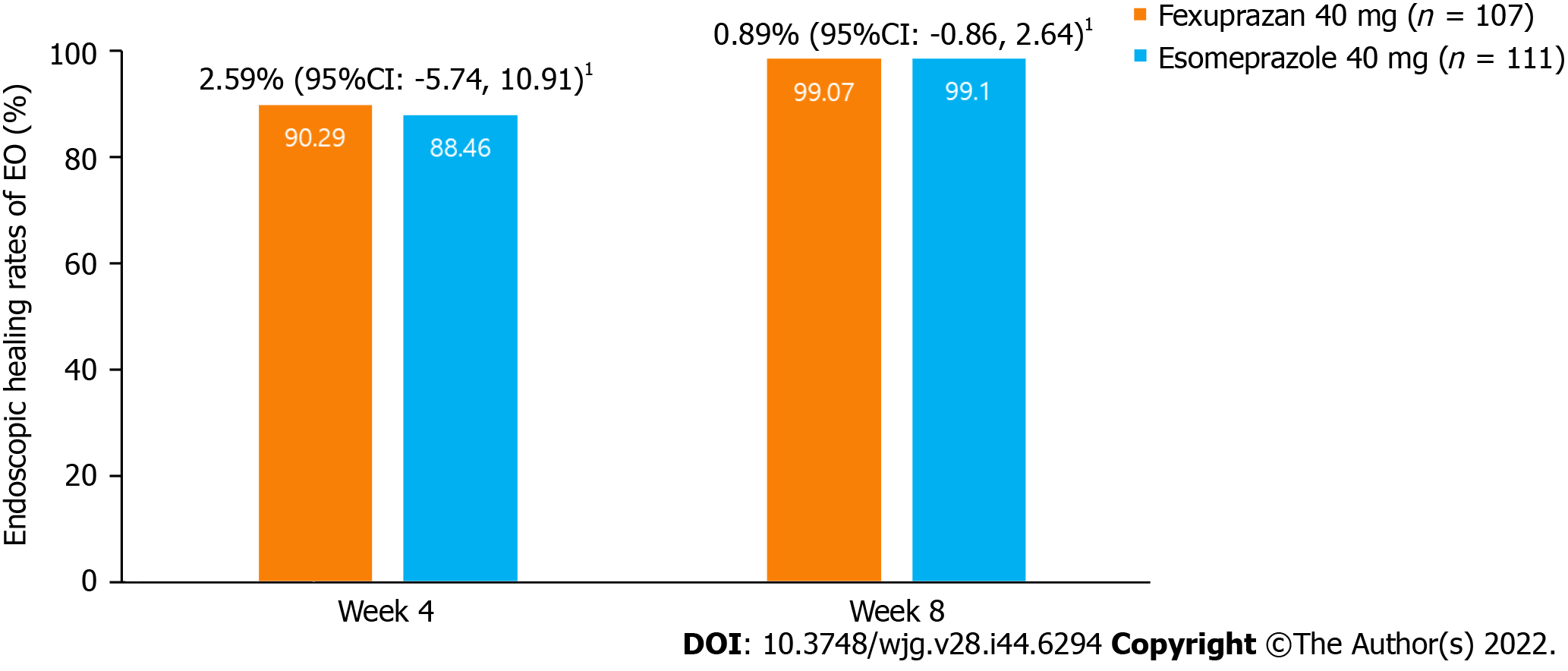
Figure 3 Erosive esophagitis healing rate at weeks 4 and 8 (per protocol set).
Erosive esophagitis (EE) healing was defined as the complete absence of mucosal breaks confirmed by the endoscopy. 1Common risk difference of the healing rate of EE between the treatment groups (two-sided 95%CI) using the Cochran-Mantel-Haenszel method adjusted by baseline Los Angeles grade. EE: Erosive esophagitis; CI: Confidence interval.
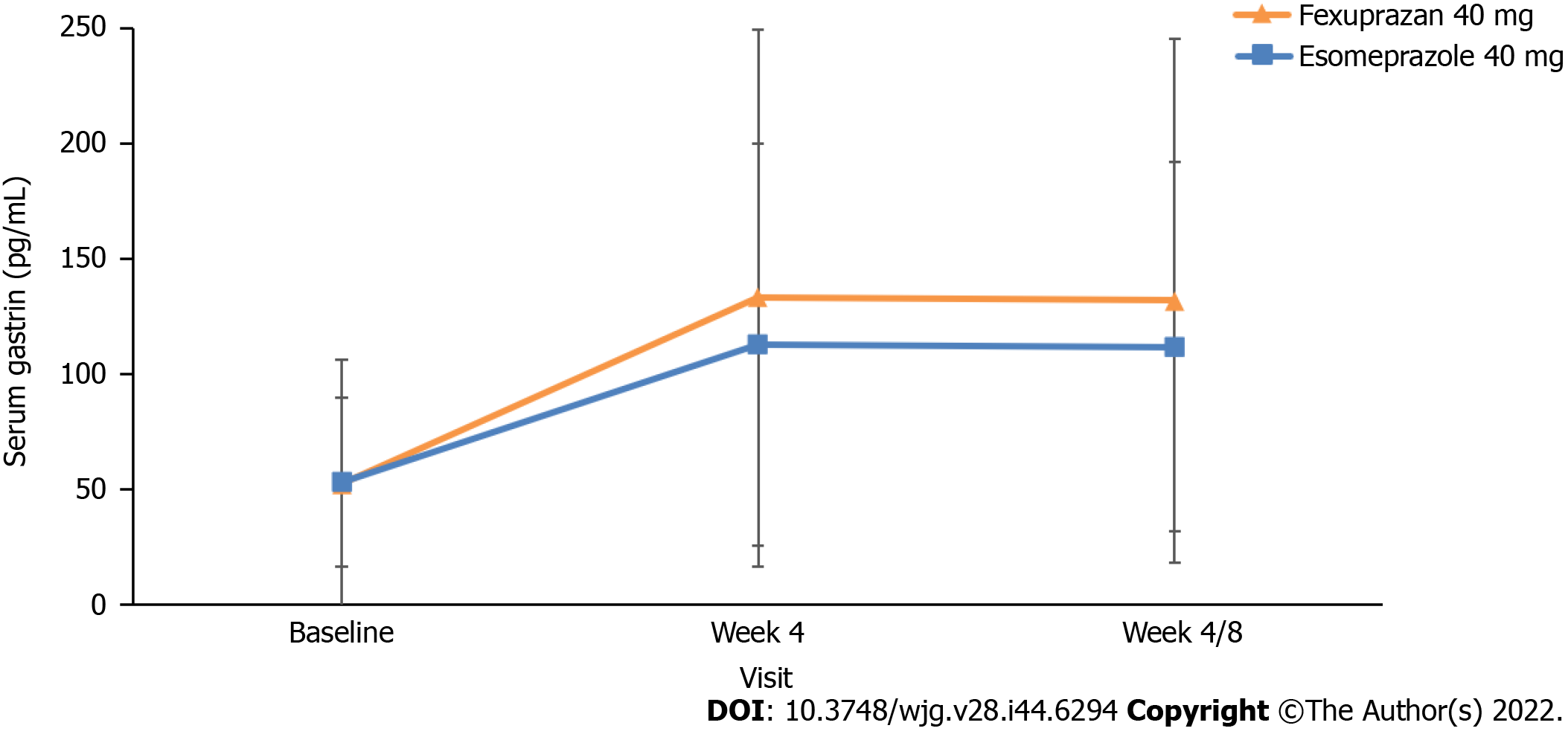
Figure 4 Changes from baseline in serum gastrin levels at weeks 4 and 8 (per-protocol set).
- Citation: Lee KN, Lee OY, Chun HJ, Kim JI, Kim SK, Lee SW, Park KS, Lee KL, Choi SC, Jang JY, Kim GH, Sung IK, Park MI, Kwon JG, Kim N, Kim JJ, Lee ST, Kim HS, Kim KB, Lee YC, Choi MG, Lee JS, Jung HY, Lee KJ, Kim JH, Chung H. Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis. World J Gastroenterol 2022; 28(44): 6294-6309
- URL: https://www.wjgnet.com/1007-9327/full/v28/i44/6294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i44.6294