Copyright
©The Author(s) 2022.
World J Gastroenterol. Oct 28, 2022; 28(40): 5784-5800
Published online Oct 28, 2022. doi: 10.3748/wjg.v28.i40.5784
Published online Oct 28, 2022. doi: 10.3748/wjg.v28.i40.5784
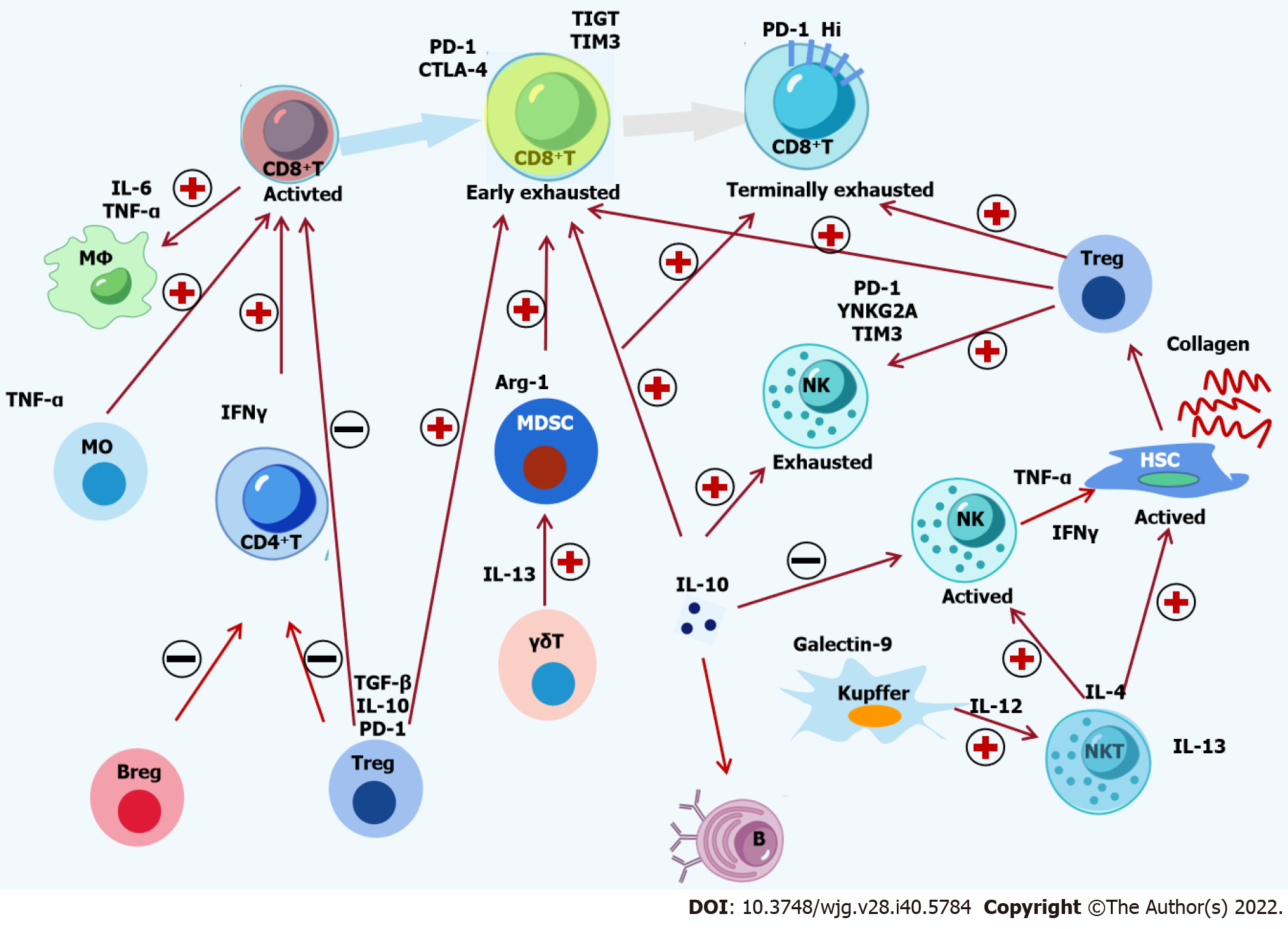
Figure 1 Crosstalk among immune cells and cytokines in hepatitis B virus infection.
The complex interactions among immune cells and cytokines in chronic hepatitis B are shown. Hepatitis B virus (HBV)-specific CD8+ T-cells are activated by monocytes and CD4+ T-cells, followed by recruitment and activation of macrophages by active CD8+ T-cells. The activation of natural killer (NK) T-cells is induced by Kupffer cells, which activate NK cells and hepatic stellate cells (HSCs). Suppressive Tregs, Bregs and Kupffer cells induce the functional impairment of CD8+ T cells, CD4+ T-cells and NK cells. Moreover, Treg cells, Kupffer cells and myeloid-derived suppressor cells can lead to the exhaustion of CD8+ T-cells and NK cells. Inflammatory and inhibitory cytokines, including Monocyte chemoattractant protein-1, tumor necrosis factor-α, interferon-γ, interleukin (IL)-4, IL-6, IL-12, IL-13, IL-17, IL-10, and transforming growth factor-β, are involved in the crosstalk among immune cells. The activation of HSCs in sinusoids is induced by a complement protein such as C5a. Finally, decreasing epigenetic modification and function of HBV-specific CD8+ T-cells inhibits the immune control of HBV. PD-1: Programmed death 1; CTLA-4: Cytotoxic T-lymphocyte antigen-4; IL: Interleukin; TNF-α: Tumor necrosis factor-α; TGF-β: Transforming growth factor β; IFN-γ: Interferon-γ; HSC: Hepatic stellate cell; MDSC: Myeloid-derived suppressor cells; NK: Natural killer.
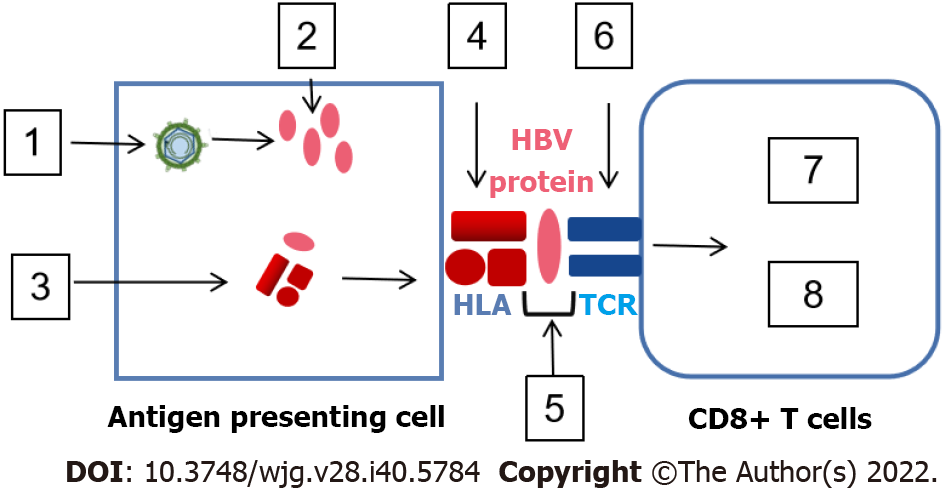
Figure 2 Mechanism of immune escape in antigen-presenting cell/hepatitis B virus special T-cell.
Hepatitis B virus (HBV)-infected hepatocytes produce various HBV antigens that are swallowed and digested by antigen-presenting cells (APCs), producing HBV peptide/human leukocyte antigen (HLA) complexes. Antigen processing escape mutants, down-regulating HLA expression and mutation of HLA binding residues may appear in APCs. The HBV peptide/HLA complexes are transferred to the surface of APC and make contact with T-cell receptor (TCR) on the surface of CD8+ T-cells. Masking HLA/TCT binding residues with N-linked glycosylation and mutation of TCR binding residues influences TCR affinity/avidity, leading to CD8+ T-cell stimulation or inhibition. The square icon displays: (1) Increase in viral antigen; (2) Antigen processing escape mutants; (3) Down-regulation of HLA expression; (4) Mutation of HLA binding residues; (5) Masking of HLA/TCT binging residues with N-linked glycosylation; (6) Mutation of TCR binding residues; (7) Stimulation induced by cytokine production and cytolytic activity; and (8) Inhibition caused by exhaustion, anergy and tolerance. HBV: Hepatitis B virus; TCR: T-cell receptor; HLA: Human leukocyte antigen.
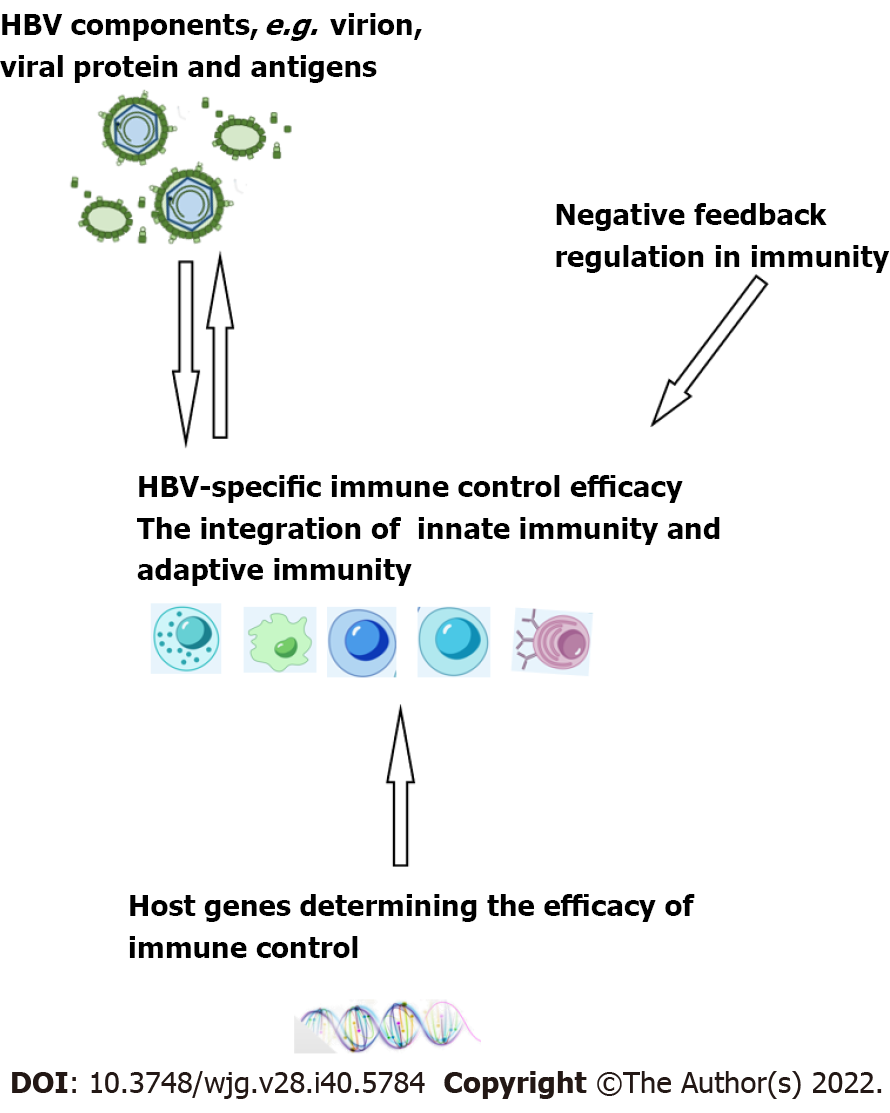
Figure 3 Interplay between hepatitis B virus-specific immune control, hepatitis B virus components, and negative feedback regulation in immunity and host genes.
HBV: Hepatitis B virus.
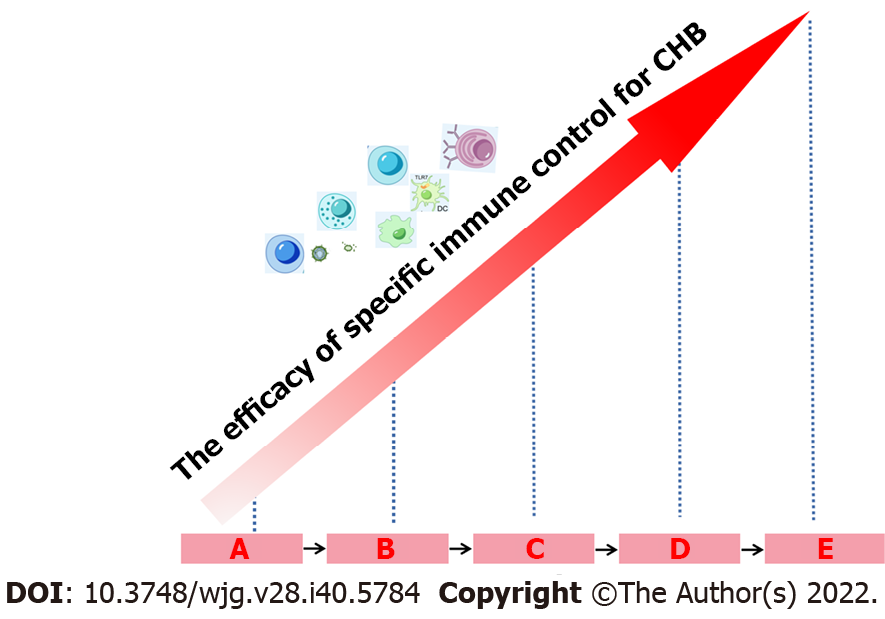
Figure 4 Interferon-α retreatment improves the efficacy of hepatitis B virus-specific immune control.
Interferon-α (IFN-α) retreatment can lead to various treatment outcomes, namely no response, hepatitis B virus (HBV) decline, partial cure and functional cure. Multiple frequencies of IFN-α treatment can potentially restore specific immune control to HBV infection and simultaneously increase the rate of partial cure and functional cure. A: Exhausted immune control to HBV before IFN-α therapy (Baseline); B: Without HBV decline following IFN-α therapy; C: HBV decline; D: Partial cure; E: Functional cure. CHB: Chronic hepatitis B.
- Citation: Yin GQ, Chen KP, Gu XC. Heterogeneity of immune control in chronic hepatitis B virus infection: Clinical implications on immunity with interferon-α treatment and retreatment. World J Gastroenterol 2022; 28(40): 5784-5800
- URL: https://www.wjgnet.com/1007-9327/full/v28/i40/5784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i40.5784