Copyright
©The Author(s) 2021.
World J Gastroenterol. Jun 28, 2021; 27(24): 3483-3501
Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3483
Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3483
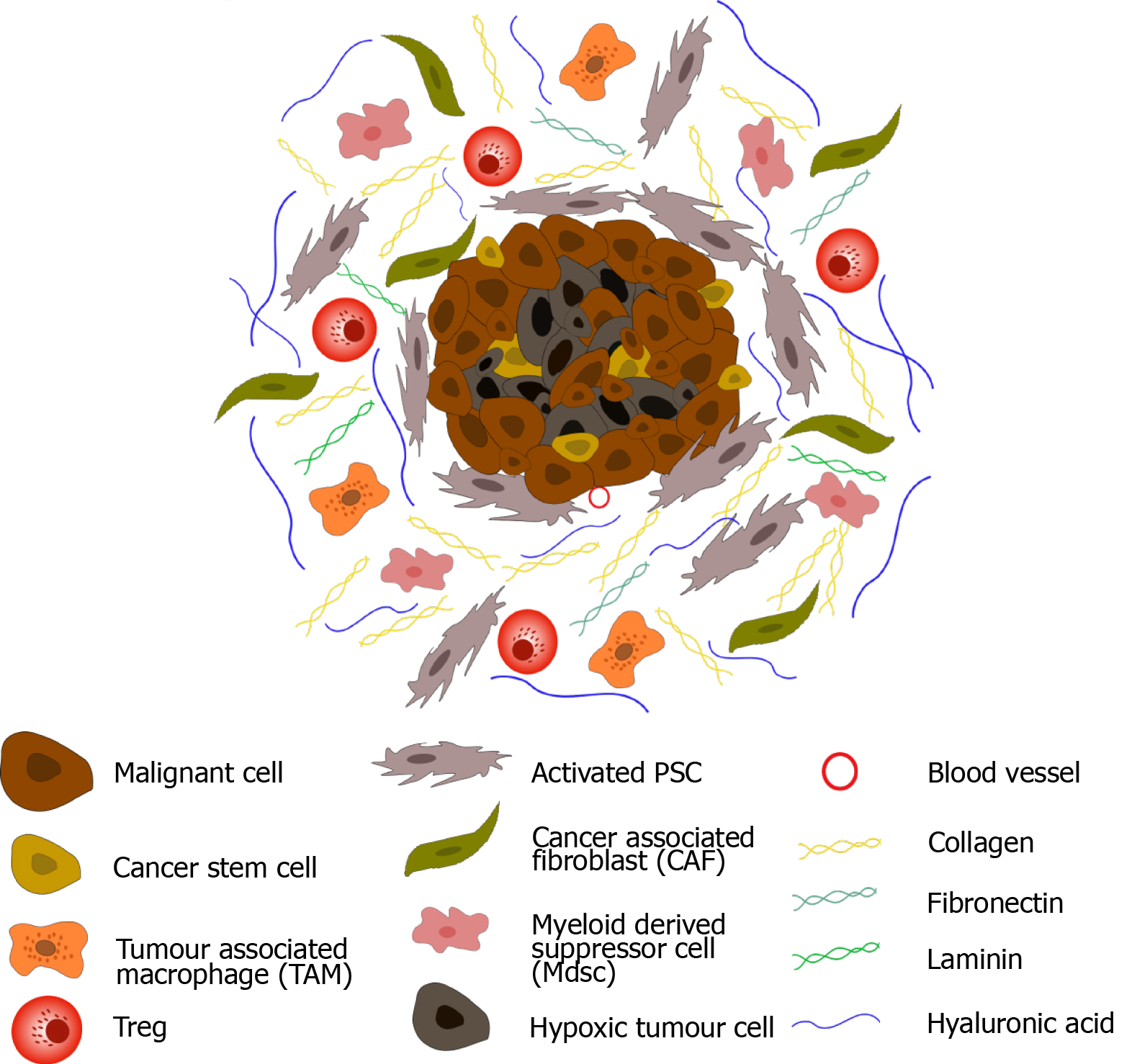
Figure 1 Pancreatic ductal adenocarcinoma tumour microenvironment.
Upon activation, pancreatic stellate cells secrete an abundance of extracellular matrix proteins including collagen, fibronectin, laminin, and hyaluronic acid, leading to dense desmoplasia. In addition, fibroblastic cells (CAFs) become active, and immune suppressive cells (myeloid-derived suppressor cells, regulatory T-cells and tumour associated macrophages) are sequestered to the tumour microenvironment (TME). Secretion of anti-angiogenic factors, in addition to dense desmoplasia, results in the development of a hypoxic TME. Cancer stem cells are also observed in pancreatic ductal adenocarcinoma.
Figure 2 Activation and cytotoxicity of natural killer cells.
Natural killer (NK) cells recognise a multitude of ligands on both healthy and transformed cells. Inhibitory receptors (red) recognise ‘self-antigens’ on healthy tissue preventing activation. However, these molecules are lost on aberrant cells as a result of viral transformation or malignancy (‘missing-self’) leading to NK cell activation. Alternatively, NK cells may become active through engagement of activating receptors (green) via stress ligands expressed on transformed cells. Binding of leukocyte function-associated antigen 1 to ICAM1 stabilises the immunological synapse between NK and target cells and ensures effective cytotoxicity. Upon activation NK cells release cytotoxic granules which contain perforin and granzymes to initiate target cell death via necrotic or apoptotic pathways. NK cells can also execute antibody dependent cellular cytotoxicity through Fc engagement of the CD16 receptor. Finally, NK cells secrete cytokines, such as interferon-γ, facilitating crosstalk between the adaptive and innate immune system, resulting in dendritic and T cell recruitment.
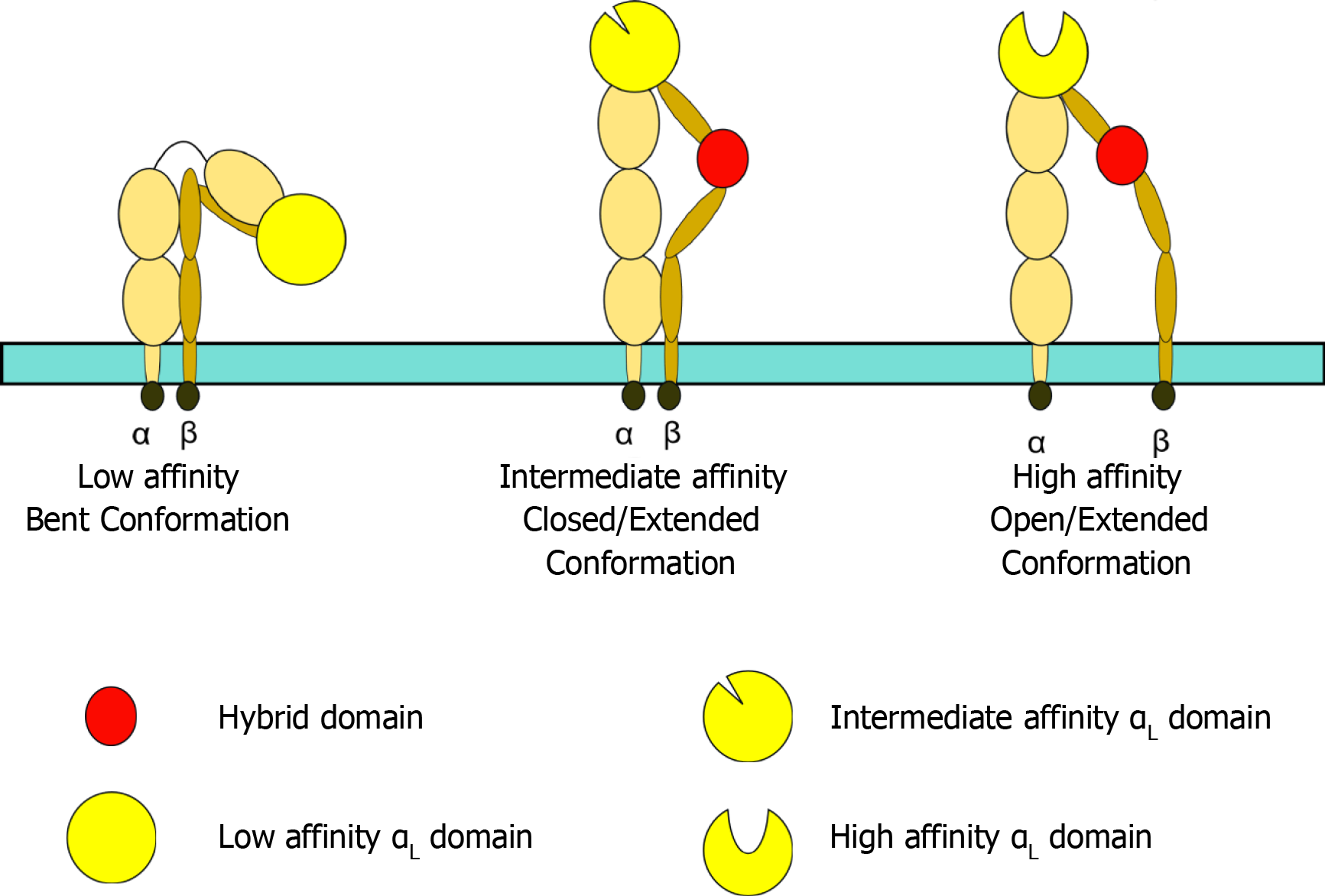
Figure 3 Leukocyte function-associated antigen 1 conformation.
Leukocyte function-associated antigen 1 (LFA-1) affinity can be altered by its conformation. When bent, LFA-1 exhibits low affinity for its ligand, ICAM1. Intermediate affinity is achieved through a closed/extended conformation, whilst an open/extended conformation results in high affinity binding to ICAM1 and generation of an effective, stable immunological synapse.
Figure 4 Cytotoxic granule convergence and microtubule organising centre polarisation.
A: Following receptor stimulation leading to natural killer (NK) cell activation, leukocyte function-associated antigen 1 engages with its ligand ICAM1 on the malignant cell, forming a stable immunological synapse; B: F-actin accumulates and polymerises at the immune synapse, forming a filamentous mesh which modulates the release of cytolytic granules. Tubulin microtubules then form from the microtubule organising centre (MTOC); C and D: Cytotoxic granules converge on the microtubules (C) and are polarised towards the MTOC where they converge (D); E: This granule movement is dependent on dynein/dynactin motor function. Dynamic rearrangement of the microtubules facilitates polarisation of MTOC towards the immunological synapse; F and G: This polarisation is stimulated via Integrin-linked kinase, paxillin, Pyk2 and RhoGEF7 signalling. Following polarisation to the immunological synapse, a subsection of cytotoxic granules fuse with the plasma membrane (F) (a process largely regulated by Munc 13-4 and Rab27a) and undergo degranulation via either complete or incomplete fusion (G); H: Cytotoxic granules which do not degranulate are recycled and are hypothesised to remain converged at the MTOC to facilitate serial NK cell killing. Granules which undergo incomplete fusion are rapidly recycled through clathrin mediated endocytosis of granule membrane proteins, further facilitating serial killing; I: Finally, the malignant cell undergoes perforin induced necrosis or granule dependent apoptosis. NK cells detach from the malignant cell and move on to the next target.
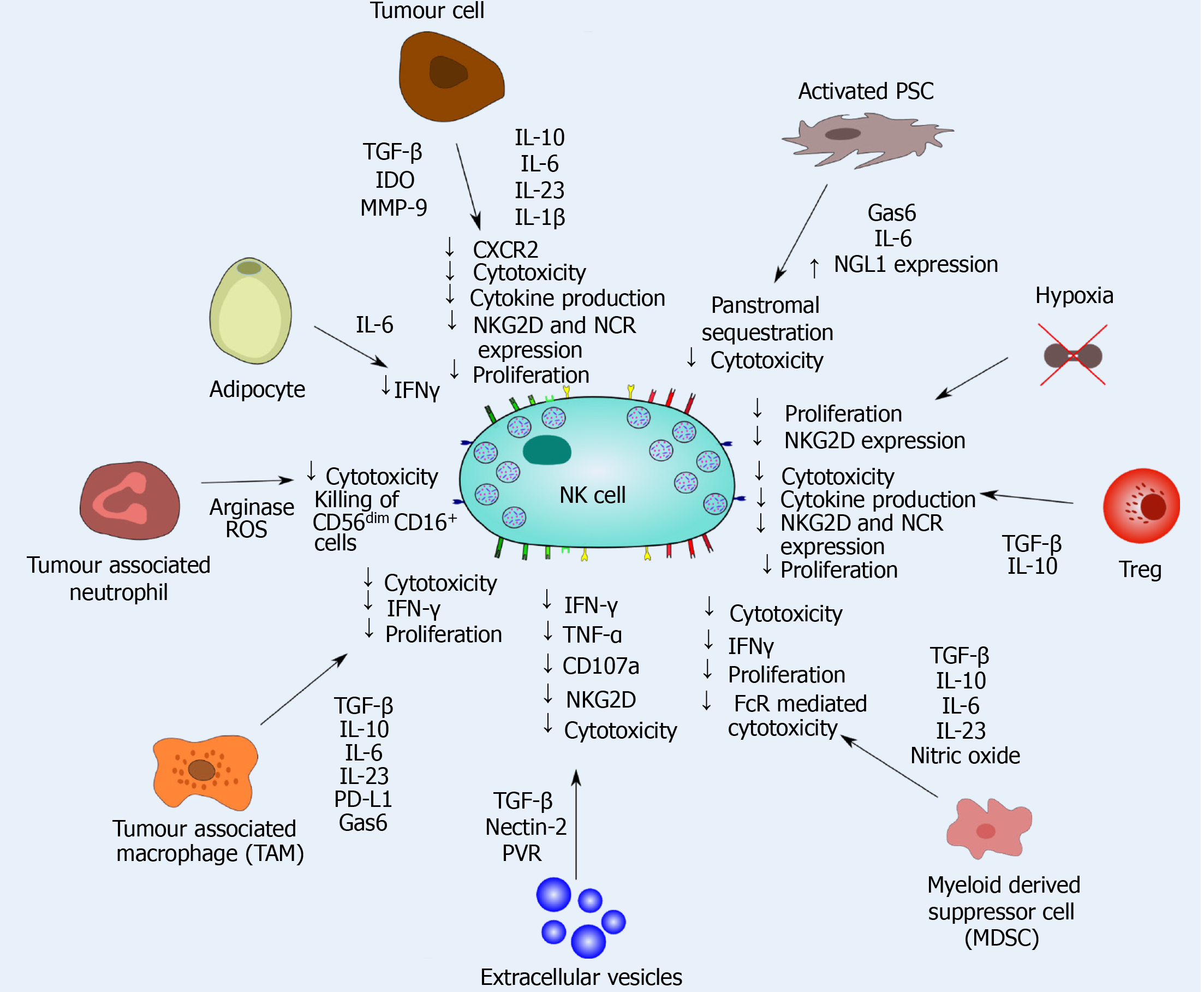
Figure 5 Natural killer cell dysfunction caused by tumoral and stromal cells in pancreatic ductal adenocarcinoma.
Natural killer (NK) cell interactions with multiple stromal and tumour cells significantly impacts the cytotoxic efficacy of NK cells in pancreatic ductal adenocarcinoma. Transforming growth factor -β, interleukin (IL)-10, IL-6, IL-23 and IL-1β release significantly dampens NK cell cytotoxicity and function, and inhibits intratumoural proliferation of NK cells. NK cell mediated cytokine release is also inhibited within the immunosuppressive tumour microenvironment. Finally, chemokine release may also sequester NK cells in the panstromal compartment, preventing engagement with tumour cells.
- Citation: Fincham REA, Delvecchio FR, Goulart MR, Yeong JPS, Kocher HM. Natural killer cells in pancreatic cancer stroma. World J Gastroenterol 2021; 27(24): 3483-3501
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3483.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3483