Copyright
©The Author(s) 2021.
World J Gastroenterol. Jun 7, 2021; 27(21): 2727-2757
Published online Jun 7, 2021. doi: 10.3748/wjg.v27.i21.2727
Published online Jun 7, 2021. doi: 10.3748/wjg.v27.i21.2727
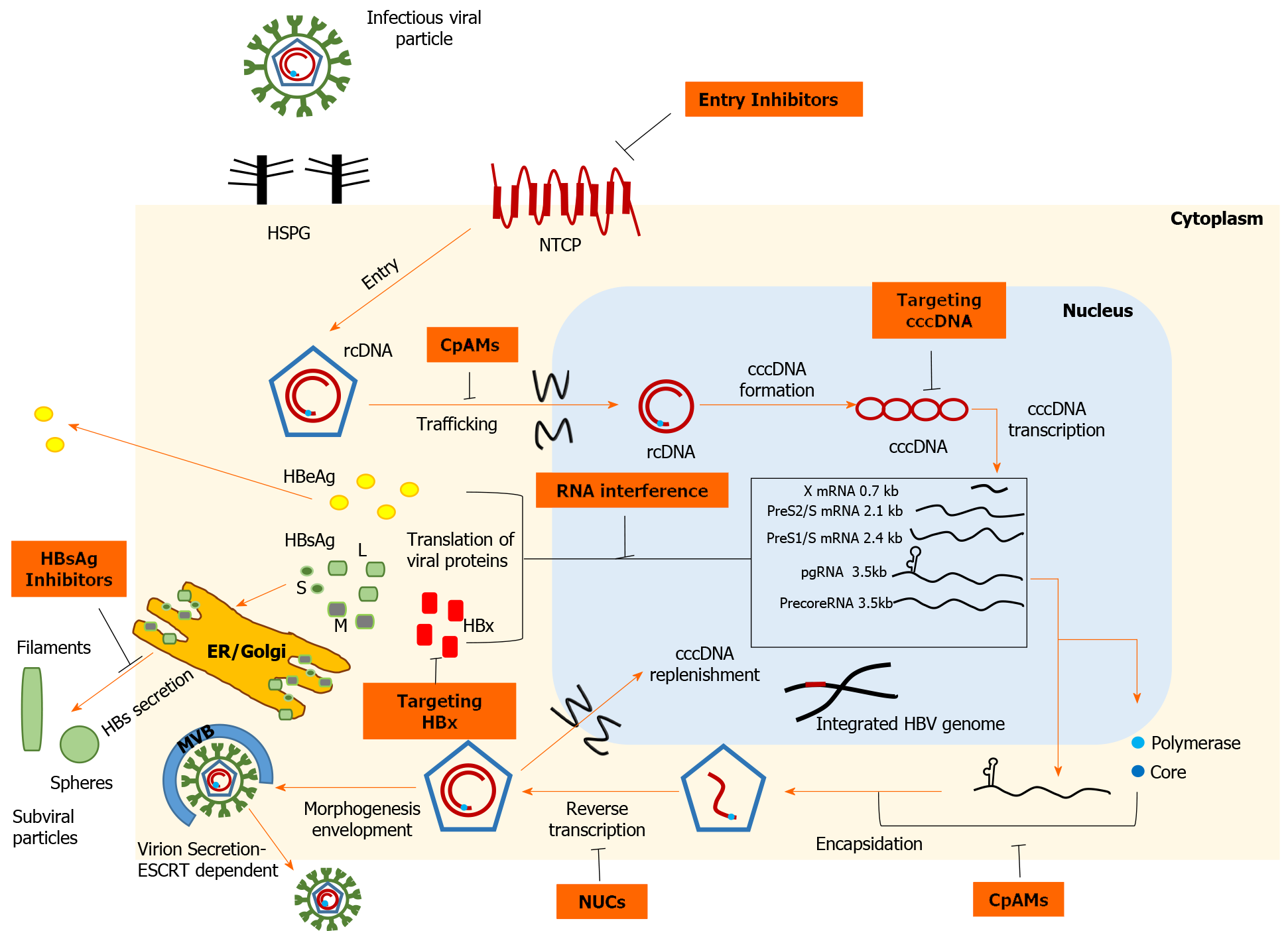
Figure 1 Schematic representation of the hepatitis B virus life cycle and the targets of direct-acting antivirals.
Viral entry can be prevented, either by disrupting viral interaction with heparan sulfate proteoglycans or by inhibiting high-affinity binding to the sodium taurocholate cotransporting polypeptide receptor. Strategies targeting covalently closed circular DNA (cccDNA) include: Prevention of cccDNA formation, cccDNA degradation or destabilization, gene editing tools to cause sequence-specific damage, and epigenetic manipulations to functionally silence cccDNA. Inhibition of the interplay between hepatitis B virus (HBV) X protein and host proteins leads to transcriptional silencing. Therapeutics based on RNA interference target viral transcripts and block HBV protein expression. Nucleoside or nucleotide drugs are approved inhibitors of reverse transcriptase. Core protein allosteric modulators interfere with the kinetics of nucleocapsid assembly/disassembly and can affect the various functions of the core protein. Hepatitis B surface antigen (HBsAg) release inhibitors limit the circulating HBsAg load. cccDNA: Covalently closed circular DNA; CpAMs: Core protein allosteric modulators; ER: Endoplasmic reticulum; ESCRT: Endosomal sorting complexes required for transport; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HBx: Hepatitis B virus X protein; HSPG: Heparan sulfate proteoglycans; NTCP: Sodium taurocholate cotransporting polypeptide; NUCs: Nucleot(s)ide analogues; rcDNA: Relaxed circular DNA.
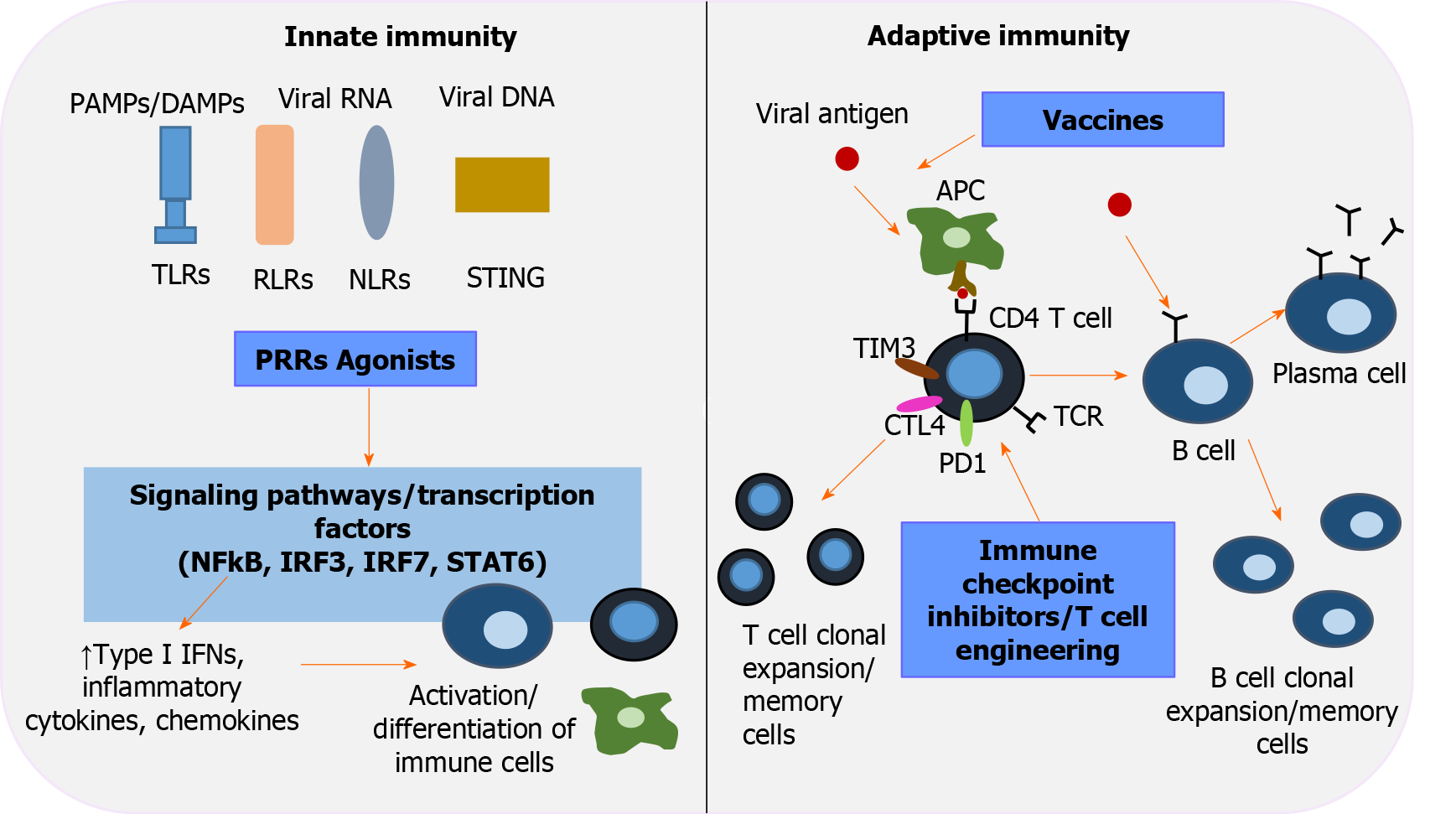
Figure 2 Immunotherapeutic interventions to revive host immunity in chronic hepatitis B virus infection.
Pattern-recognition receptors (PRRs), including toll-like receptors, retinoic acid-inducible gene-I-like receptors, nucleotide-binding oligomerization domain-like receptors, stimulator of interferon genes, are key players in innate immunity and the first line of defense that recognizes pathogen-associated molecular patterns/damage-associated molecular patterns. Activation of PRRs by their corresponding agonists triggers transduction signals and transcription factors [nuclear factor-κB, interferon regulatory factor (IRF) 3, IRF 7, signal transducer and activator of transcription 6], which in turn upregulate type interferons, inflammatory cytokines and chemokines, leading to well-orchestrated immune cell differentiation. Immune checkpoint inhibitors aim to restore T-cell function by inhibiting negative regulators of T-cell activation (programmed cell death protein 1, cytotoxic T-lymphocyte-associated protein 4, T-cell immunoglobulin and mucin domain-3). Adoptive transfer of genetically engineered T -cells is an alternative strategy to elicit potent hepatitis B virus - specific T-cell responses. The role of therapeutic vaccination in overcoming exhausted cellular and humoral responses is currently under investigation. An efficient vaccine repairs function and induces antigen- presenting cells to activate the two arms of adaptive immunity: polyclonal and multispecific CD4+ and CD8+ T-cell responses, and well-regulated B cells that differentiate into plasma cells and secrete neutralizing antibodies. IFNs: Interferons; IRF: Interferon regulatory factor; NF-κΒ: Nuclear factor-κB; NLRs: Nucleotide-binding oligomerization domain-like receptors; PAMPS: Pathogen-associated molecular patterns; PD-1: Programmed cell death protein 1; PRRs: Pattern-recognition receptors; RLRs: Retinoic acid-inducible gene-I-like receptors; STAT6: Signal transducer and activator of transcription 6; STING: Stimulator of interferon genes; TIM3: T-cell immunoglobulin and mucin domain-3; TLRs: Toll-like receptors.
- Citation: Tsounis EP, Tourkochristou E, Mouzaki A, Triantos C. Toward a new era of hepatitis B virus therapeutics: The pursuit of a functional cure. World J Gastroenterol 2021; 27(21): 2727-2757
- URL: https://www.wjgnet.com/1007-9327/full/v27/i21/2727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i21.2727