Copyright
©The Author(s) 2021.
World J Gastroenterol. Mar 14, 2021; 27(10): 976-989
Published online Mar 14, 2021. doi: 10.3748/wjg.v27.i10.976
Published online Mar 14, 2021. doi: 10.3748/wjg.v27.i10.976
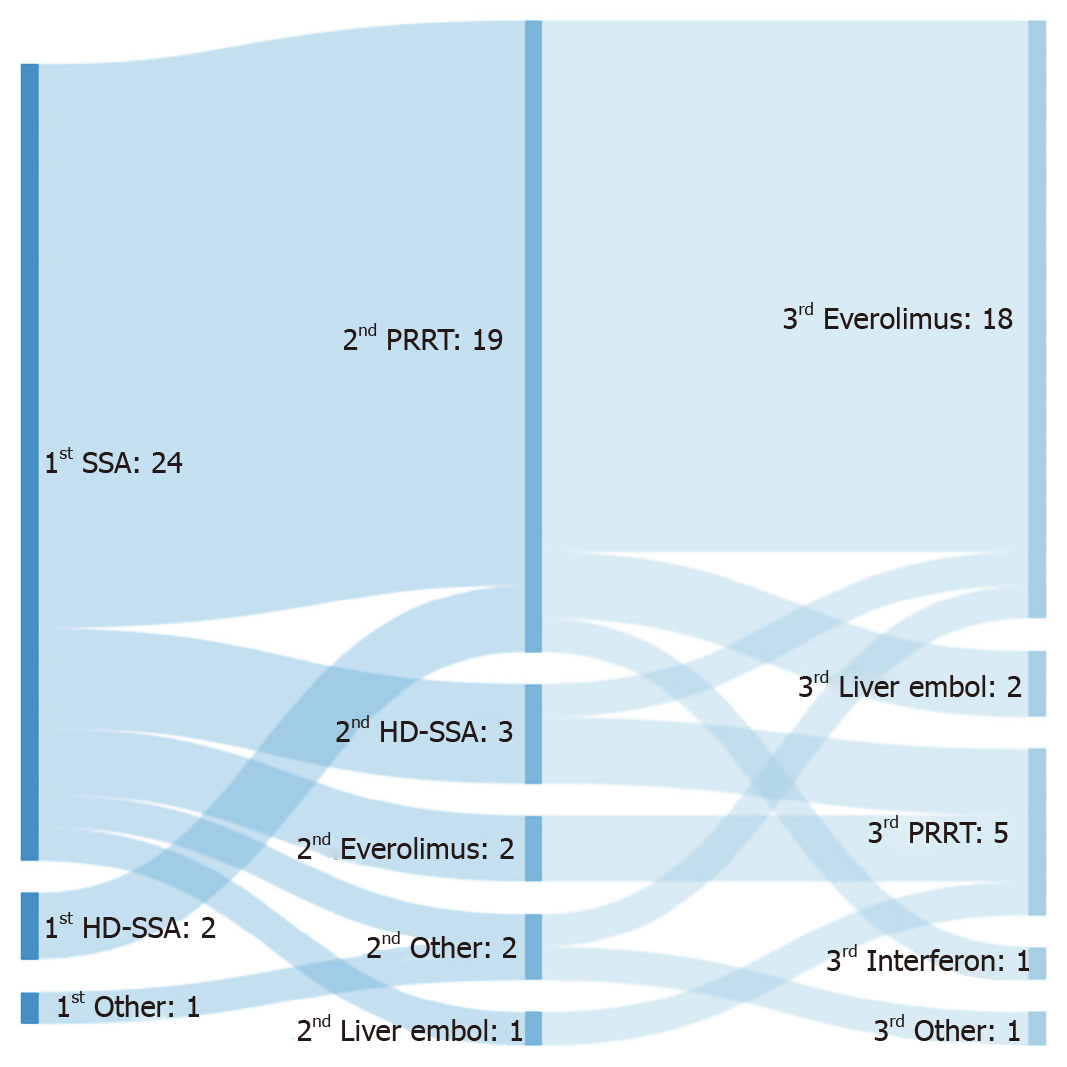
Figure 1 Summary of treatment predominant flow by line of therapy.
SSA: Somatostatin analogues; PRRT: Peptide receptor radionuclide therapy; Liver embol: Liver embolization; HD-SSA: High-dose of somatostatin analogues.
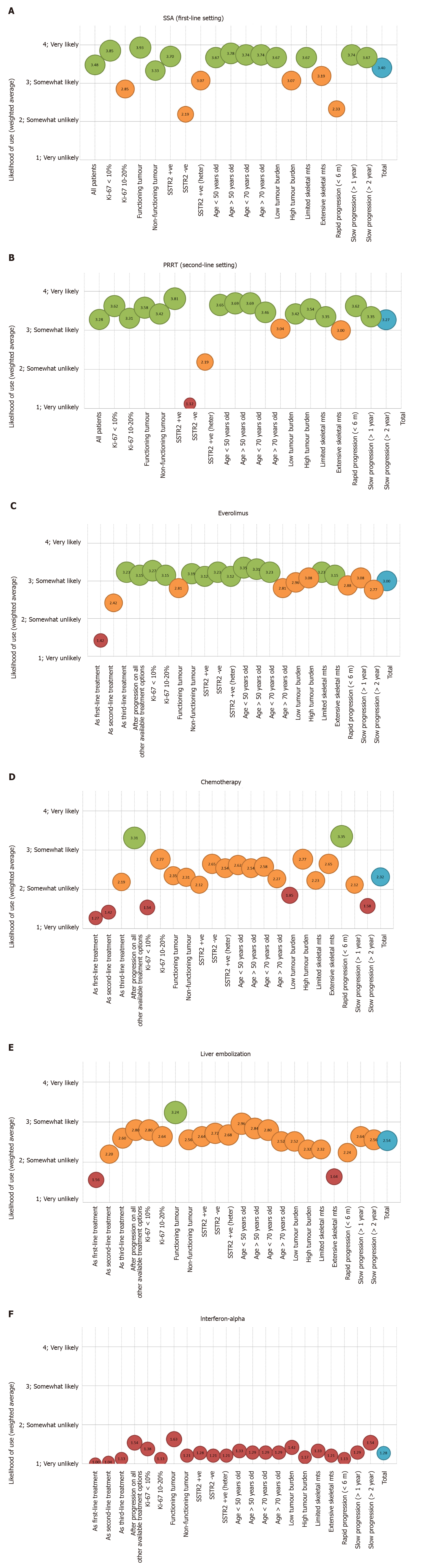
Figure 2 Factors associated with treatment decision making process.
A: Factors associated with use of somatostatin analogues in the first-line setting; B: Factors associated with use of peptide receptor radionuclide therapy in the second-line setting; C: Factors associated with use of everolimus; D: Factors associated with use of chemotherapy; E: Factors associated with use of liver embolization; F: Factors associated with use of Interferon-alpha. SSA: Somatostatin analogues; SSTR2 –ve: Somatostatin receptor positive disease (imaging based); SSTR2 +ve: Somatostatin receptor negative disease (imaging based); heter: Heterogeneous; mts: Metastases; m: months; TOTAL: Represents the average of all factors; PRRT: Peptide receptor radionuclide therapy.
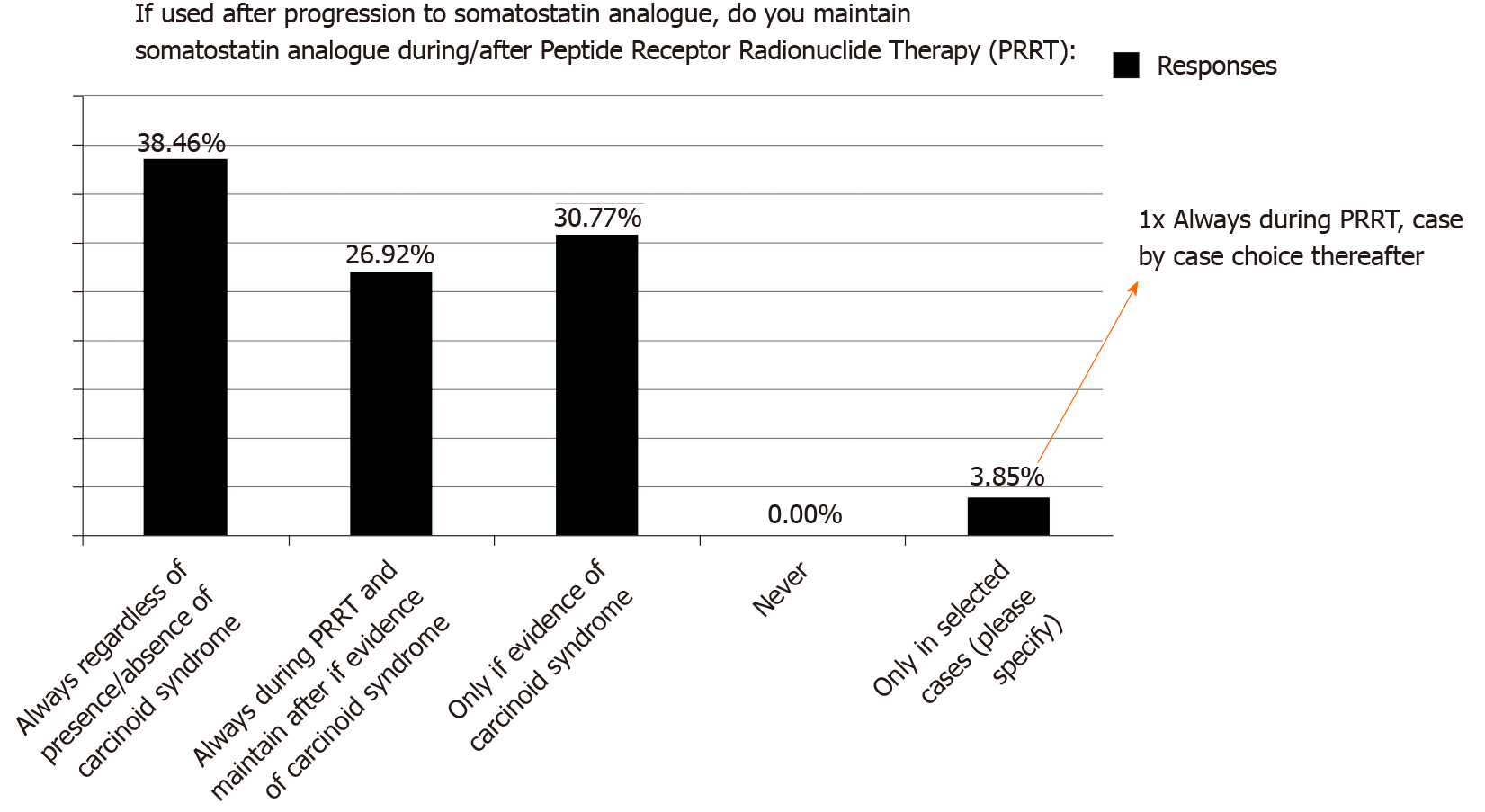
Figure 3
Ongoing controversies around use of peptide receptor radionuclide therapy in current practice.
- Citation: Lamarca A, Cives M, de Mestier L, Crona J, Spada F, Öberg K, Pavel M, Alonso-Gordoa T. Advanced small-bowel well-differentiated neuroendocrine tumours: An international survey of practice on 3rd-line treatment. World J Gastroenterol 2021; 27(10): 976-989
- URL: https://www.wjgnet.com/1007-9327/full/v27/i10/976.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i10.976