Copyright
©The Author(s) 2020.
World J Gastroenterol. Oct 14, 2020; 26(38): 5759-5783
Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5759
Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5759
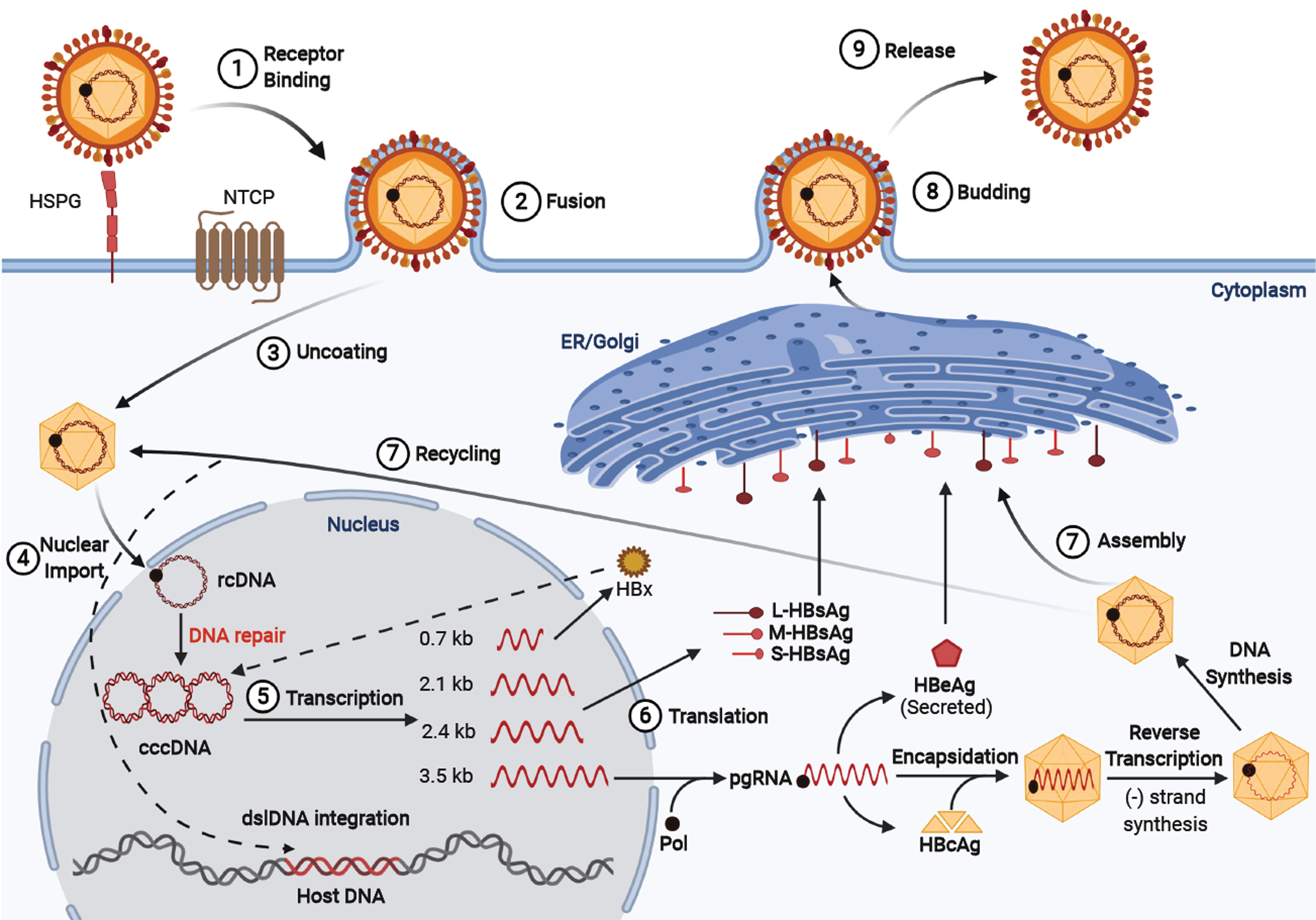
Figure 1 Hepatitis B virus life cycle.
Viral entry is mediated by low-affinity binding of the Pre-S1 protein to the heparin sulfate proteoglycan receptor, followed by binding to the sodium-taurocholate co-transporting polypeptide to facilitate entry. The nucleocapsid is transported from the cytoplasm to the nucleus where the relaxed circular DNA (rcDNA) genome is converted into the persistent covalently closed circular DNA (cccDNA) form. Viral mRNA is then transcribed from the cccDNA genome and translated at the rough endoplasmic reticulum. The greater than genome length pregenomic RNA is transported to the cytoplasm, encapsidated by the hepatitis B virus core protein and reverse transcribed by the hepatitis B virus polymerase to produce rcDNA or double-stranded linear DNA. The core particles can then obtain their envelope proteins at the endoplasmic reticulum to be excreted out of the cell, or the core particles containing double-stranded linear DNA can relocate into the nucleus and integrate into the host genome, and the rcDNA can be recycled intracellularly to replenish the cccDNA pool.
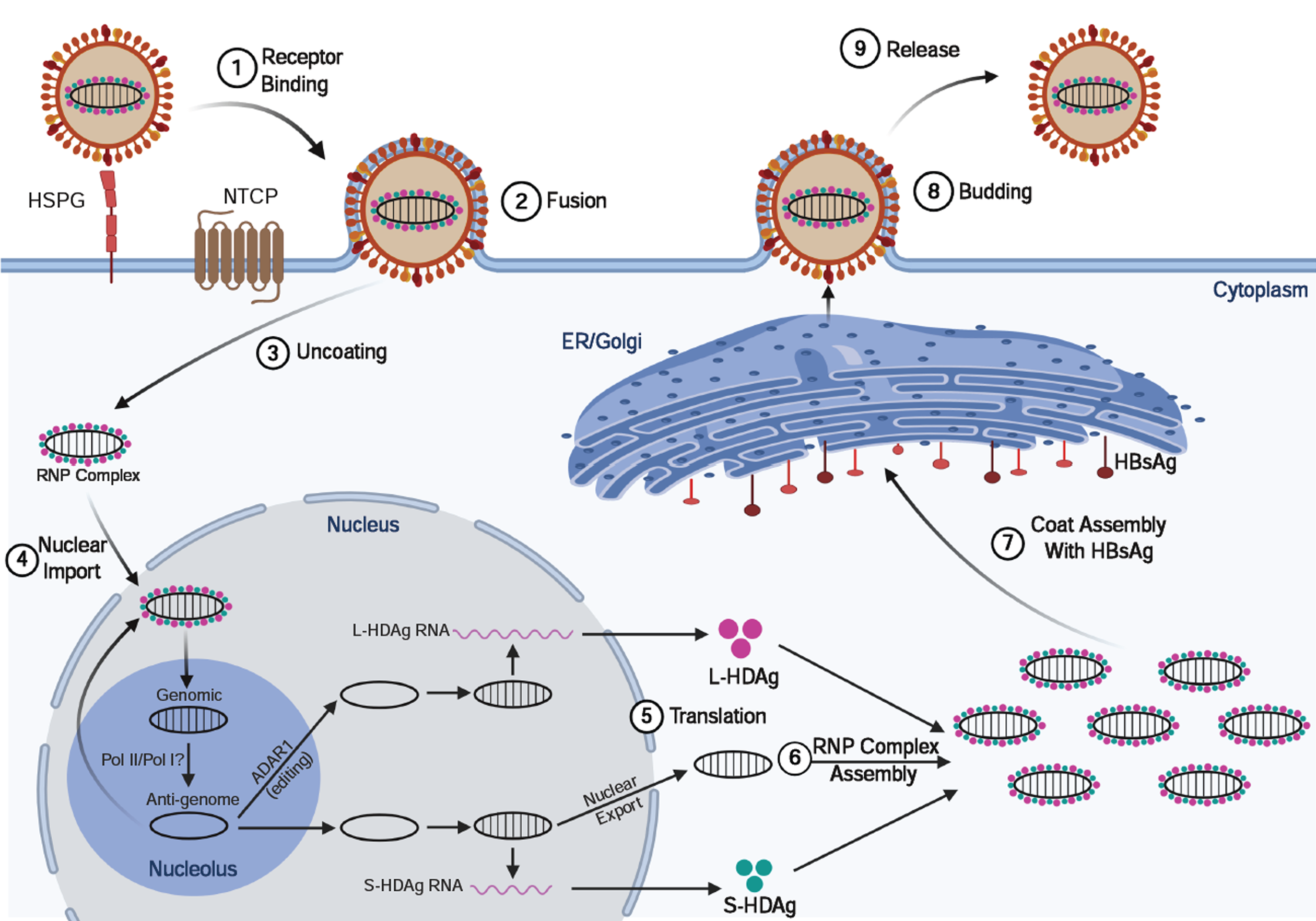
Figure 2 Hepatitis delta virus lifecycle.
Viral entry is mediated (like hepatitis B virus) by low-affinity binding of the Pre-S1 protein to the heparin sulfate proteoglycan receptor, followed by binding to the sodium-taurocholate co-transporting polypeptide to facilitate entry. Following uncoating, the ribonucleoprotein (RNP) complex consisting of negative-sense single-stranded RNA genome plus the small and large hepatitis delta virus (HDV) antigens (L-HDAg/S-HDAg) are transported to the nucleus. Within the nucleolus, HDV RNA is replicated using a double rolling circle amplification to form the positive-sense anti-genomic RNA and more genomic RNA. From the amplification process, the genomic RNA is transported out of the nucleolus and into the nucleus where it can be transcribed to produce the S-HDAg transcript or undergo A to I editing by ADARI to produce the L-HDAg RNA. Once the RNA transcripts are exported out of the nucleus, translation machinery produces the S-HDAg and L-HDAg which associate with the genomic HDV RNA to produce the RNP complex. The RNP complex passes through the endoplasmic reticulum and Golgi apparatus to obtain its coat and are then released out of the cell to infect neighboring hepatocytes. ER: Endoplasmic reticulum; NTCP: Sodium-taurocholate co-transporting polypeptide; HSPG: Heparin sulfate proteoglycan receptor; RNP: Ribonucleoprotein.
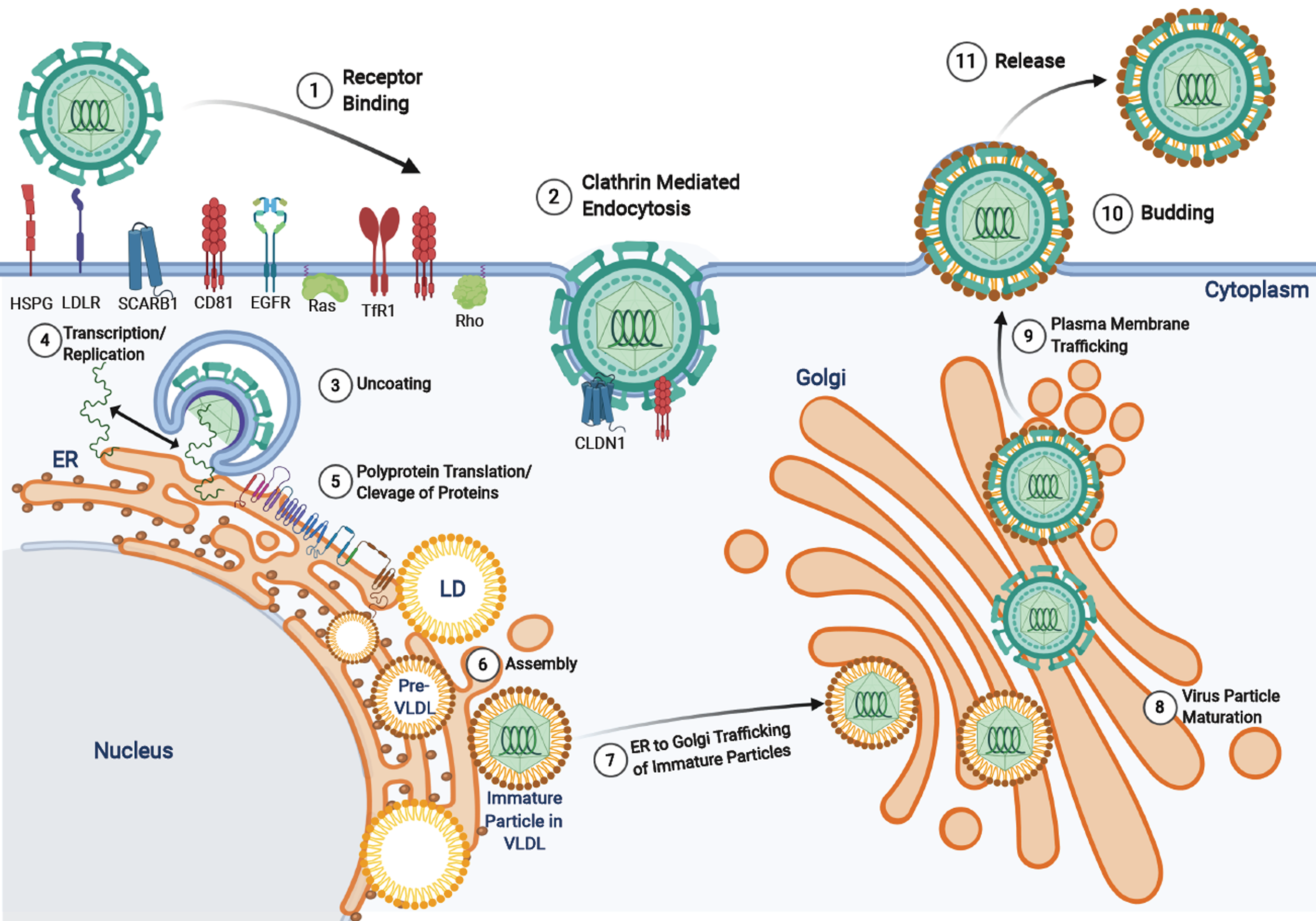
Figure 3 Hepatitis C virus life cycle.
Hepatitis C virus entry is facilitated by a variety of receptors and signaling pathways (described in[37]). Upon viral entry, the positive sense RNA genome is released into the cytoplasm from endosomal acidification. The viral RNA undergoes replication and translation at the rough endoplasmic reticulum to produce a single polyprotein chain at the endoplasmic reticulum membrane that is cleaved by viral and host proteases into 10 different viral proteins (structural and non-structural). The virus particles are assembled on lipid droplets and associate with very-low-density lipoproteins which mature at the Golgi apparatus and are released via the secretory pathway. HSPG: Heparin sulfate proteoglycan receptor; LD: Lipid droplets; VLDL: Very-low-density lipoproteins.
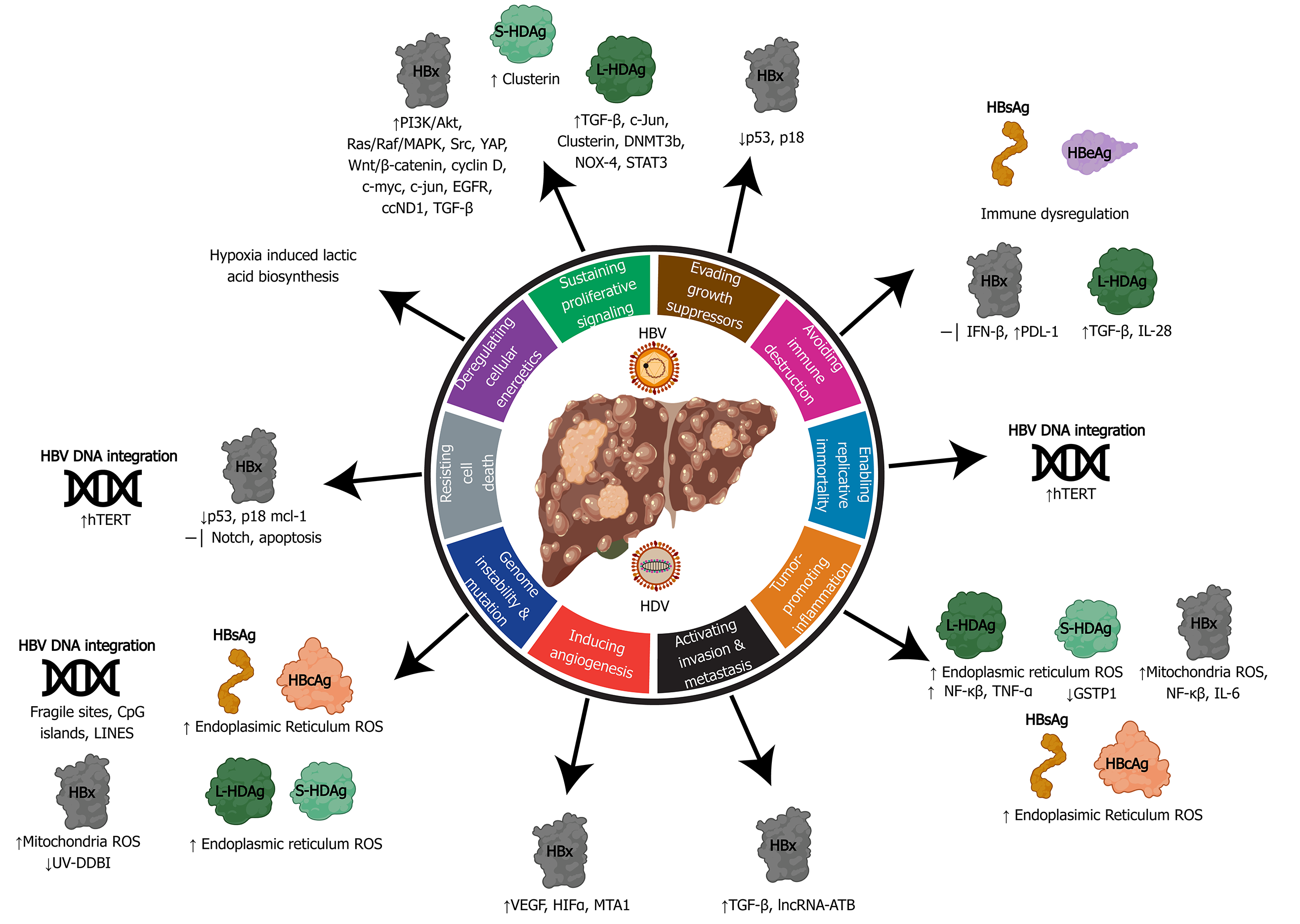
Figure 4 Relating the hallmarks of cancer to the molecular mechanisms of hepatitis B virus and delta virus hepatocarcinogenesis.
Hepatitis B virus can activate all ten hallmarks of cancer using viral proteins (HBx, HBsAg, HBeAg, HBcAg) and DNA integration. Hepatitis delta virus has been linked to four hallmarks, primarily through molecular mechanisms manipulated by the large and small hepatitis delta virus antigens (L-HDAg and S-HDAg). HBV: Hepatitis B virus; HDV: Hepatitis delta virus; ER: Endoplasmic reticulum; ROS: Reactive oxygen species.
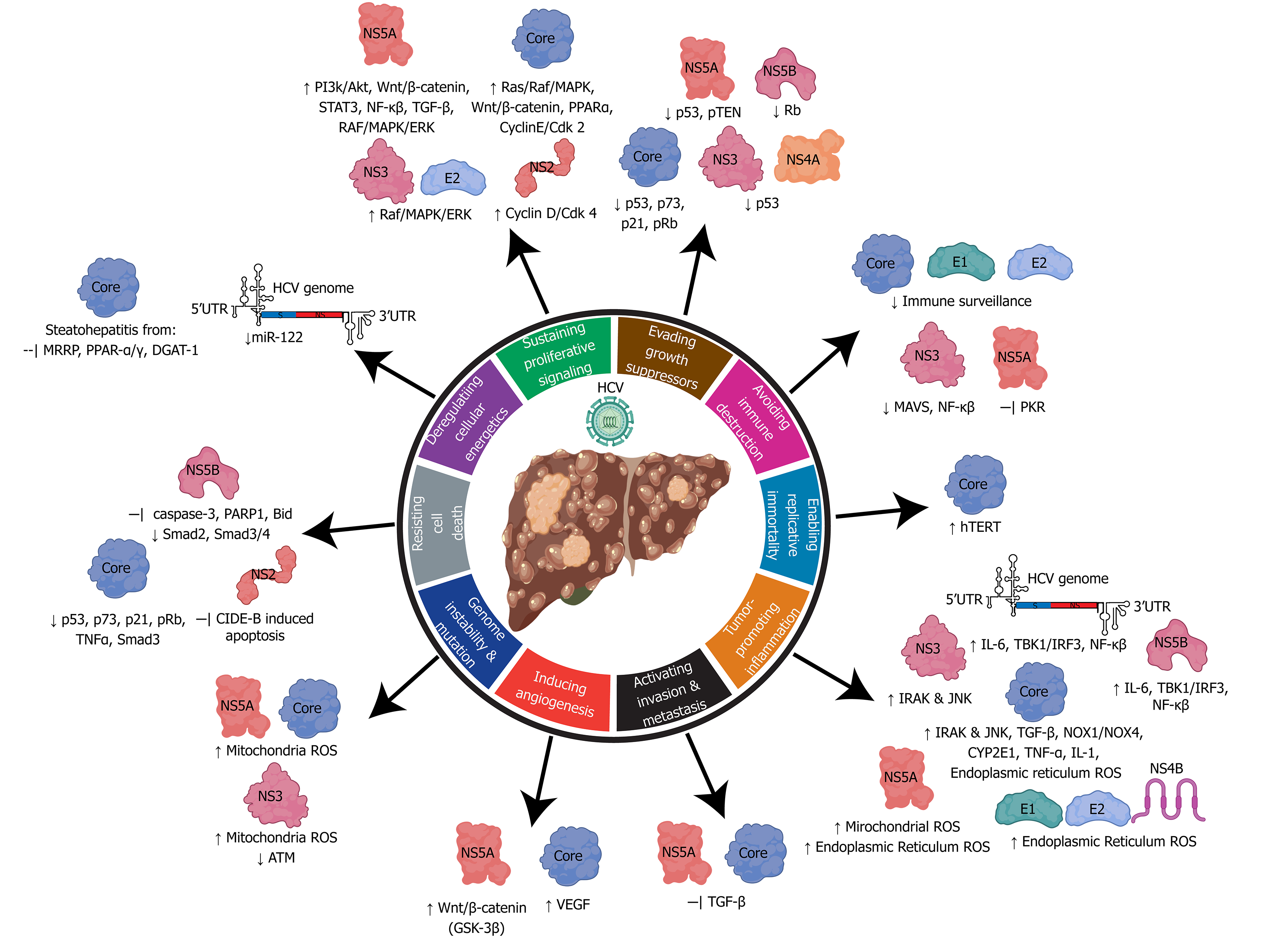
Figure 5 Relating the hallmarks of cancer to the molecular mechanisms of hepatitis C virus hepatocarcinogenesis.
Hepatitis C virus uses its RNA genome and many viral associated structural and non-structural proteins to alter cellular pathways to influence all ten hallmarks of cancer. HCV: Hepatitis C virus.
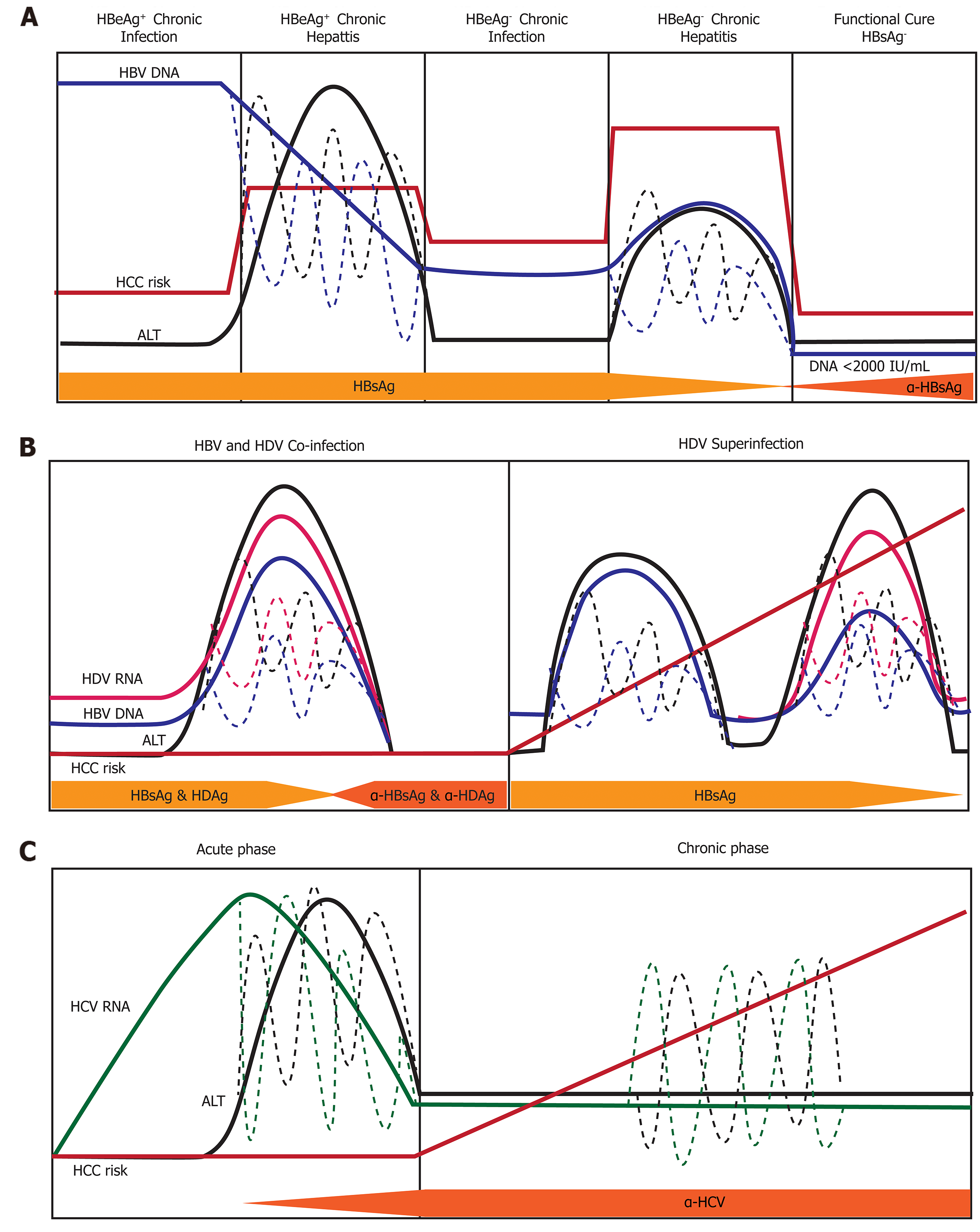
Figure 6 Natural history of infection with hepatitis B, delta, or C virus.
Variations in hepatitis B virus (HBV) DNA, hepatitis C virus RNA, hepatitis delta virus (HDV) RNA, and ALT levels indicated by dashed lines. A: Natural history of chronic Hepatitis B virus infection. There are five phases of infection HBeAg+ chronic infection, HBeAg+ chronic hepatitis, HBeAg- chronic infection, and HBeAg- phase. Each clinical phase is defined by a host immune response with respect to HBV viral activity; B: Natural history of HDV infection in either HBV co-infection or HDV superinfection when the individual is a chronic carrier of HBV; and C: Natural history of Hepatitis C virus infection. There are two main phases of infection acute infection and chronic infection. HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HDV: Hepatitis delta virus; HCV: Hepatitis C virus.
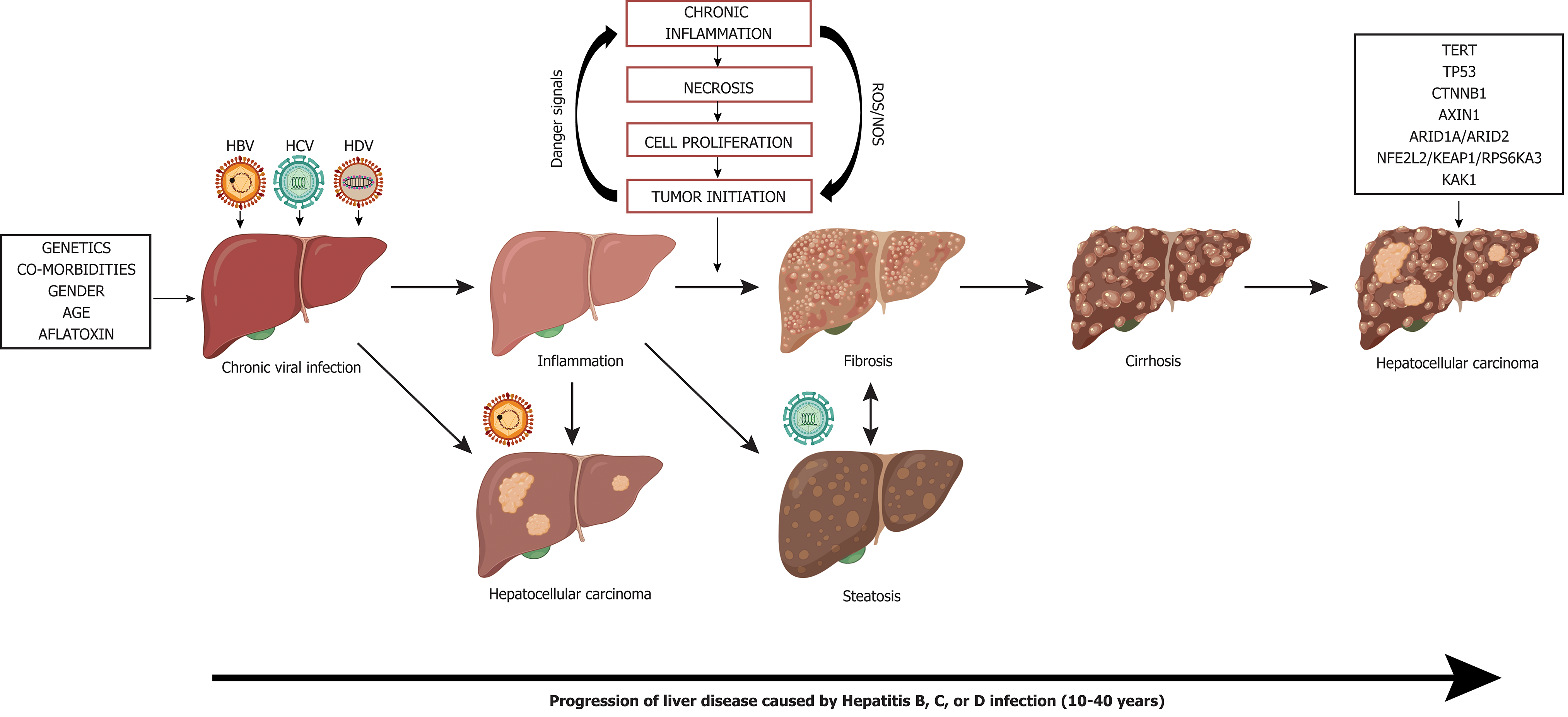
Figure 7 Liver disease progression to hepatocellular carcinoma from chronic viral hepatitis infection.
Genetics, co-morbidities, gender, age, and aflatoxin exposure influence liver disease progression along with chronic viral infection with hepatitis B, C, and/or delta virus. Cirrhosis is the greatest risk factor for development of hepatocellular carcinoma, however, hepatocellular carcinoma in the context of chronic Hepatitis B virus infection can occur in the absence of cirrhosis. Chronic hepatitis C infection can lead to steatohepatitis, which can accelerate fibrosis and cirrhosis. Superinfection with Hepatitis delta virus in individuals who have chronic Hepatitis B virus infection creates an accelerated disease course leading to liver failure and/or hepatocellular carcinoma. Many driver mutations (telomerase reverse transcriptase, TP53, CTNNB1, AXIN1, ARID1A/ARID2, NFE2L2/KEAP1/RPS6KA3, KAK1) can occur as liver disease progresses to hepatocellular carcinoma and can lead to accelerated disease progression. TERT: Telomerase reverse transcriptase; HBV: Hepatitis B virus; HDV: Hepatitis delta virus; HCV: Hepatitis C virus.
- Citation: D'souza S, Lau KCK, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol 2020; 26(38): 5759-5783
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5759