Copyright
©The Author(s) 2019.
World J Gastroenterol. Aug 7, 2019; 25(29): 3870-3896
Published online Aug 7, 2019. doi: 10.3748/wjg.v25.i29.3870
Published online Aug 7, 2019. doi: 10.3748/wjg.v25.i29.3870
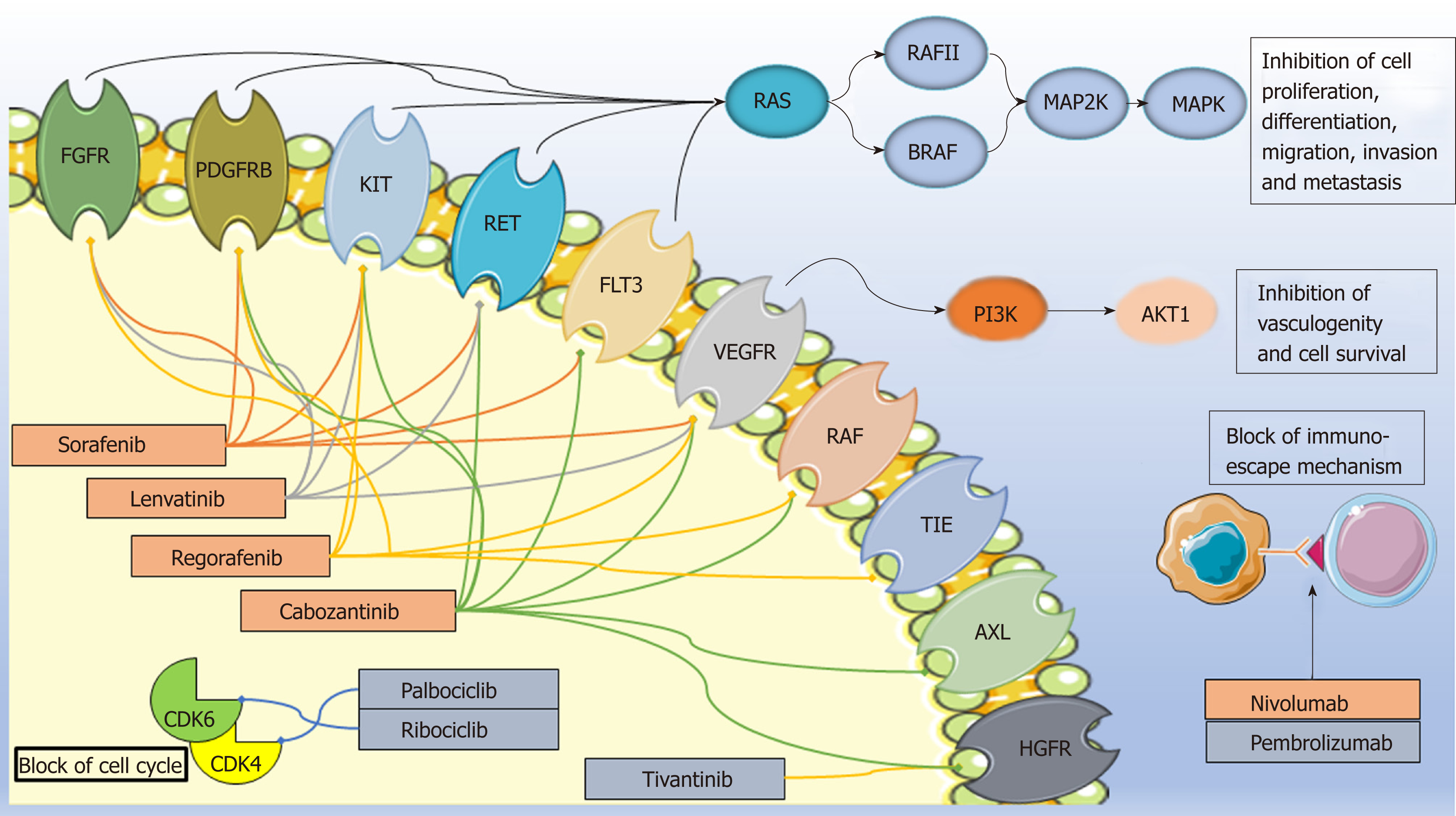
Figure 1 Mechanisms of action of the drugs covered in the text.
Approved drugs are in the orange box while those under approval are in the grey box. Image created with Servier Medical Art (https://smart.servier.com/). FGFR1: Fibroblast growth factor receptor; PDGFR: Platelet-derived growth factor receptor; FLT3: Fms-related tyrosine kinase 3; VEGFR: Vascular endothelial growth factor receptor; TIE: Tyrosine kinase with immunoglobulin-like and EGF-like domains; HGFR: Hepatocyte growth factor receptor; PD1: Programmed cell death protein-1; PDL1/2: programmed cell death protein ligand 1/2; CDK: Cyclin-dependent kinases.
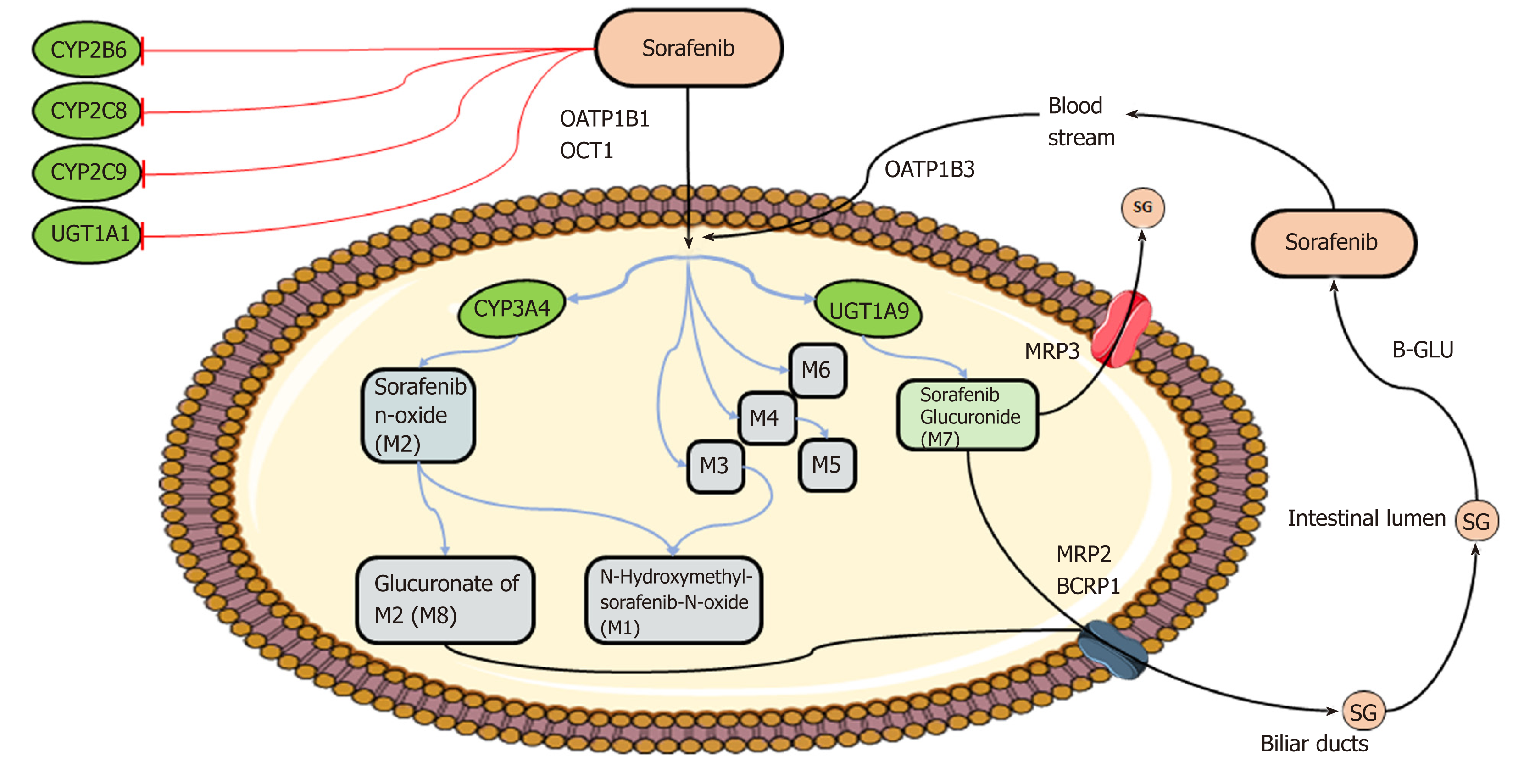
Figure 2 Schematic overview of sorafenib metabolism.
Briefly, after oral administration, sorafenib enters hepatocytes by anion transporter family member (OATP1B, encoded by SLCO1B)-type carriers and cation transporter-1 (OCT1, encoded by SLC22A1). Within the hepatocytes, sorafenib undergoes phase I cytochrome P450 3A4 (CYP3A4)- and phase II UDP glucuronosyltransferase 1A9 (UGT1A9)-mediated metabolism to form M1-8 metabolites and sorafenib glucuronide (SG). After conjugation, SG is extensively secreted into the bile by a process that is mainly mediated by multidrug resistance protein (MRP) 2 (encoded by ABCC2) and breast cancer resistance protein BCRP (encoded by ABCG2) and into the bloodstream by MRP3 (encoded by ABCC3). A fraction of SG enters the intestinal lumen, where it could be a substrate for bacterial β-glucuronidases (B-GLU) that regenerate the parental drug sorafenib, which reenters the systemic circulation through the OATP1B3 carrier. CYP2B6, CYP2C8, CYP2C9, and UGT1A1 may interfere with sorafenib metabolism, being inhibited by sorafenib (see text for details). Image created with Servier Medical Art (https://smart.servier.com/).
- Citation: De Mattia E, Cecchin E, Guardascione M, Foltran L, Di Raimo T, Angelini F, D’Andrea M, Toffoli G. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol 2019; 25(29): 3870-3896
- URL: https://www.wjgnet.com/1007-9327/full/v25/i29/3870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i29.3870