Copyright
©The Author(s) 2019.
World J Gastroenterol. May 28, 2019; 25(20): 2430-2441
Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2430
Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2430
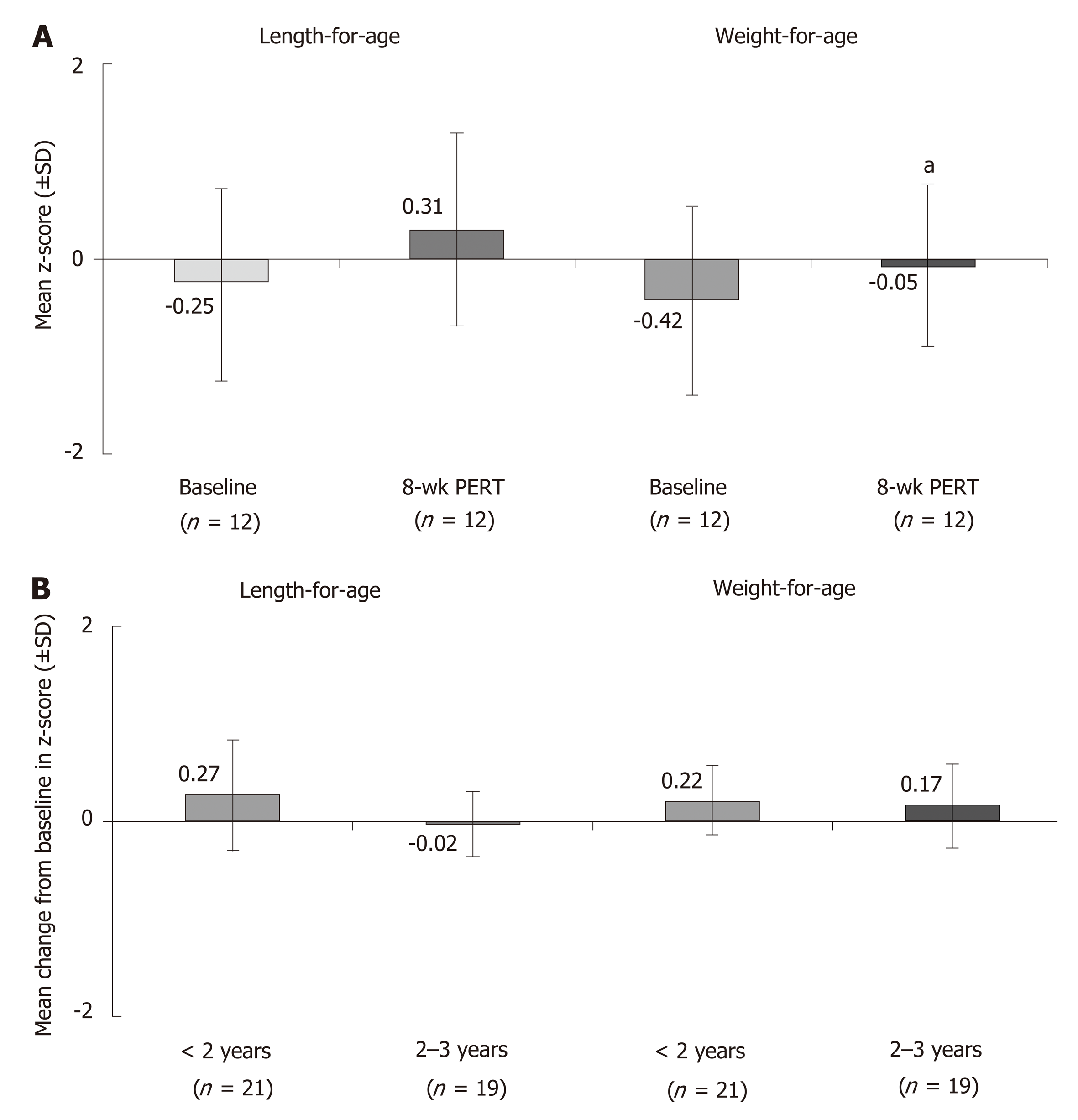
Figure 1 Effects of pancreatic enzyme replacement therapy on body weight in children with cystic fibrosis and pancreatic exocrine insufficiency.
A: Age-adjusted z-scores1 for length and weight at baseline and after 8 wk of pancreatic enzyme replacement therapy (PERT) in children < 2 years of age[27]; B: mean change from baseline after 12 wk of PERT in age-adjusted z-scores1 for length and weight in children 1 mo to < 4 years of age[28]. 1Z-scores are commonly used to assess growth percentiles and compare patients of different ages and at different rates of growth. It indicates the number of standard deviation away from the mean. aP < 0.05. PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
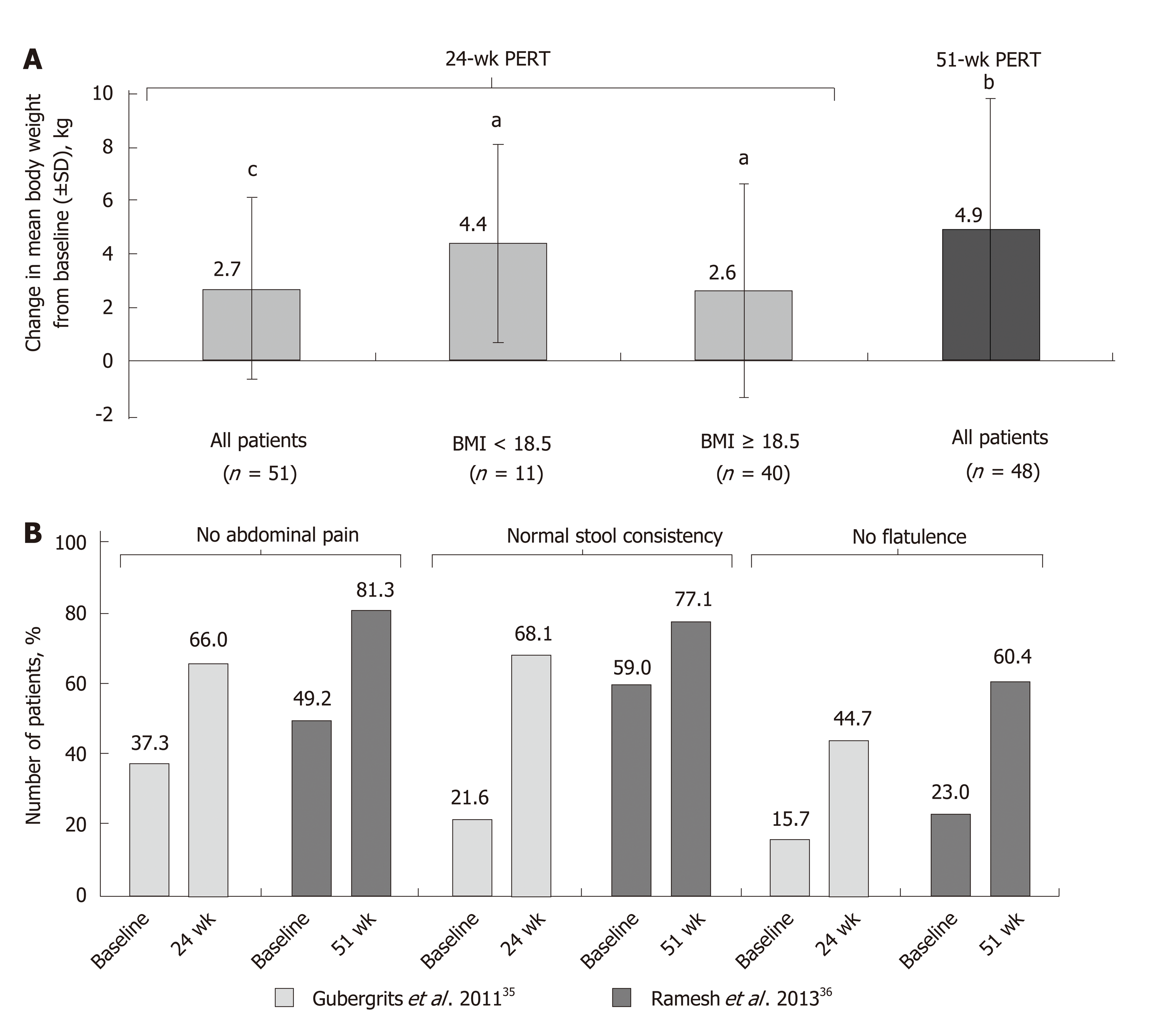
Figure 2 Mean change from baseline in body weight[35,36] (A); improvement in clinical symptoms of pancreatic exocrine insufficiency in patients with cystic fibrosis following long-term pancreatic enzyme replacement therapy[35,36] (B).
aP < 0.05; bP < 0.001; cP < 0.0001 vs baseline. BMI: Body mass index; PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
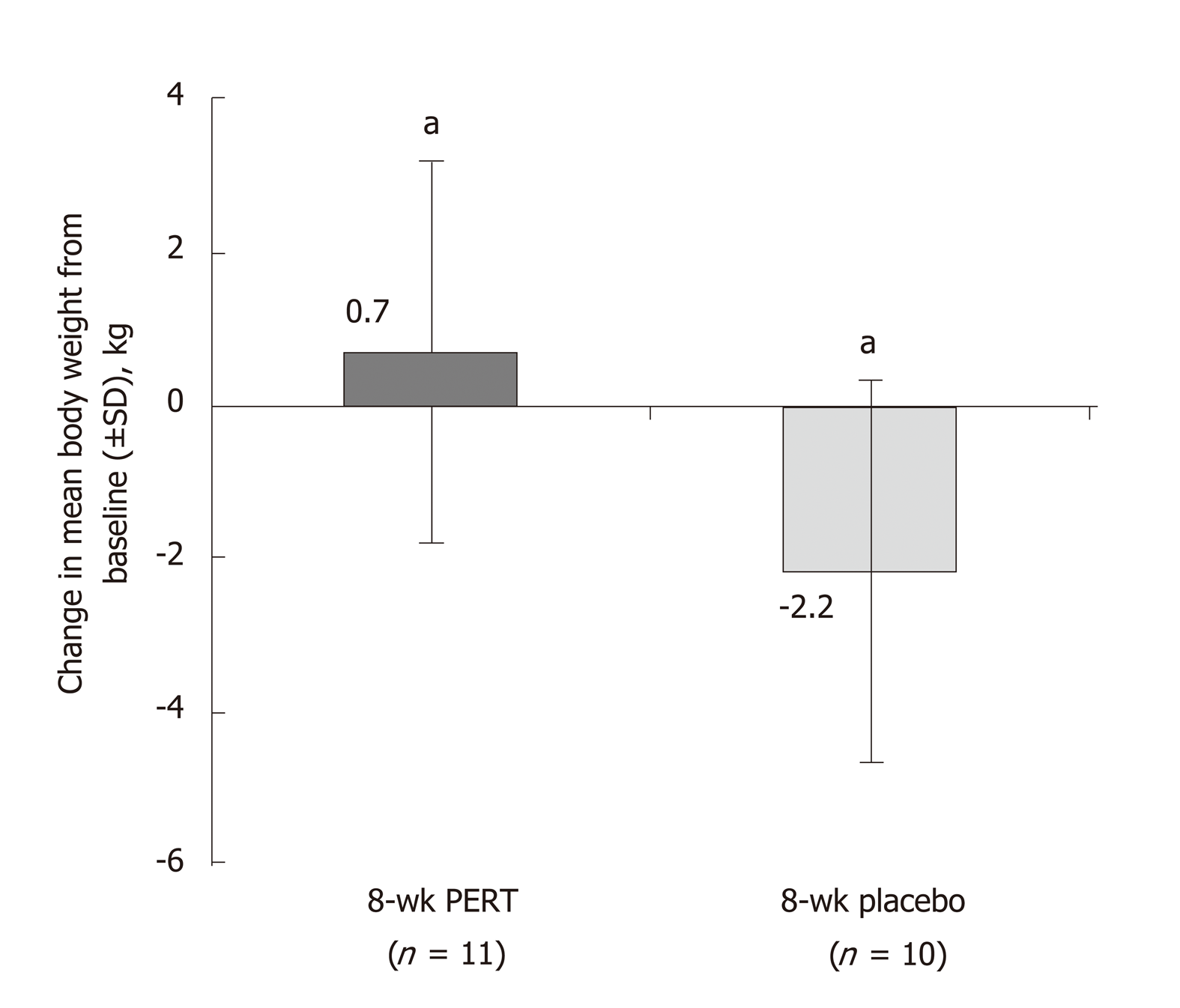
Figure 3 Effects of 8 wk of pancreatic enzyme replacement therapy on body weight of patients with unresectable pancreatic cancer[37].
aP < 0.02. PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
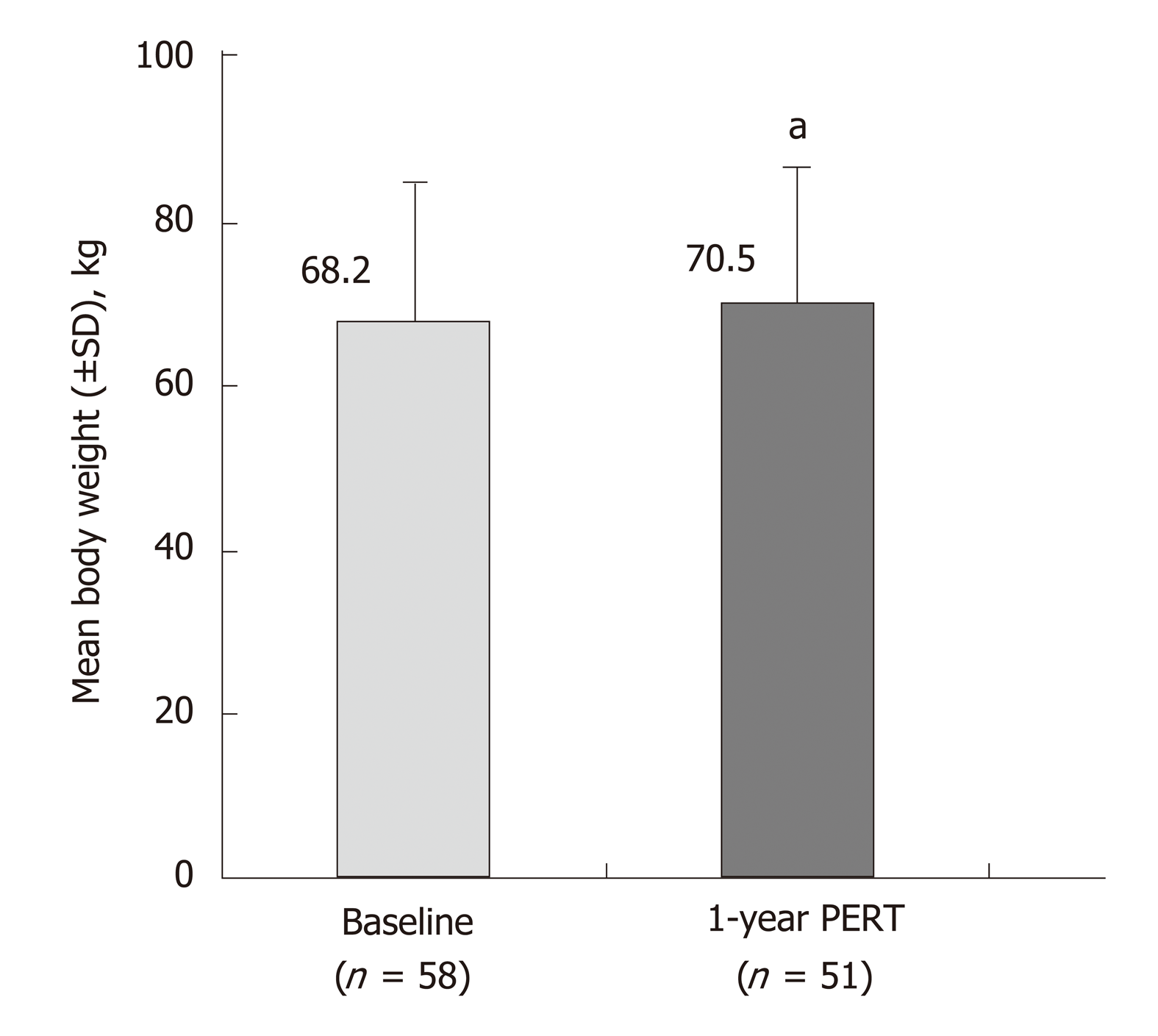
Figure 4 Effects of 51 wk of pancreatic enzyme replacement therapy on body weight of patients following total/partial resection[39].
aP < 0.05 vs Baseline. PERT: Pancreatic enzyme replacement therapy; SD: Standard deviation.
- Citation: Layer P, Kashirskaya N, Gubergrits N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World J Gastroenterol 2019; 25(20): 2430-2441
- URL: https://www.wjgnet.com/1007-9327/full/v25/i20/2430.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i20.2430