Copyright
©The Author(s) 2019.
World J Gastroenterol. Jan 7, 2019; 25(1): 138-150
Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.138
Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.138
Figure 1 Circulating tumor cell subpopulations classified by categorical markers.
(Red dots: epithelial biomarker expression; green dots: mesenchymal biomarker expression). A: Epithelial circulating tumor cells (E-CTCs); B: Mesenchymal CTCs (M-CTC); C: Biophenotypic epithelial/mesenchymal CTCs (E/M-CTCs).
Figure 2 The presence of circulating tumor cells was correlated with a poor patient prognosis.
A: Circulating tumor cell (CTC) count by AJCC stage of disease. B: CTC status in the lymph node invasion-negative group and invasion-positive group. C: CTC status in the metastasis-negative group and metastasis-positive group. D: Receiver operating characteristic curve analysis showing the performance of CTCs in predicting metastatic disease. E: Kaplan-Meier survival curves showing different overall survival in groups of pancreatic ductal adenocarcinoma (PDAC) patients with ≥ 6 or < 6 total CTCs. F: Kaplan-Meier survival curves showing different progression-free survival in groups of PDAC patients with ≥ 6 or < 6 total CTCs. G: White blood cell, neutrophil, lymphocyte, and monocyte counts in CTC-positive and CTC-negative patients. H: Neutrophil-to-lymphocyte ratio in CTC-positive and CTC-negative patients. aP < 0.05; bP < 0.01. CTC: Circulating tumor cells.
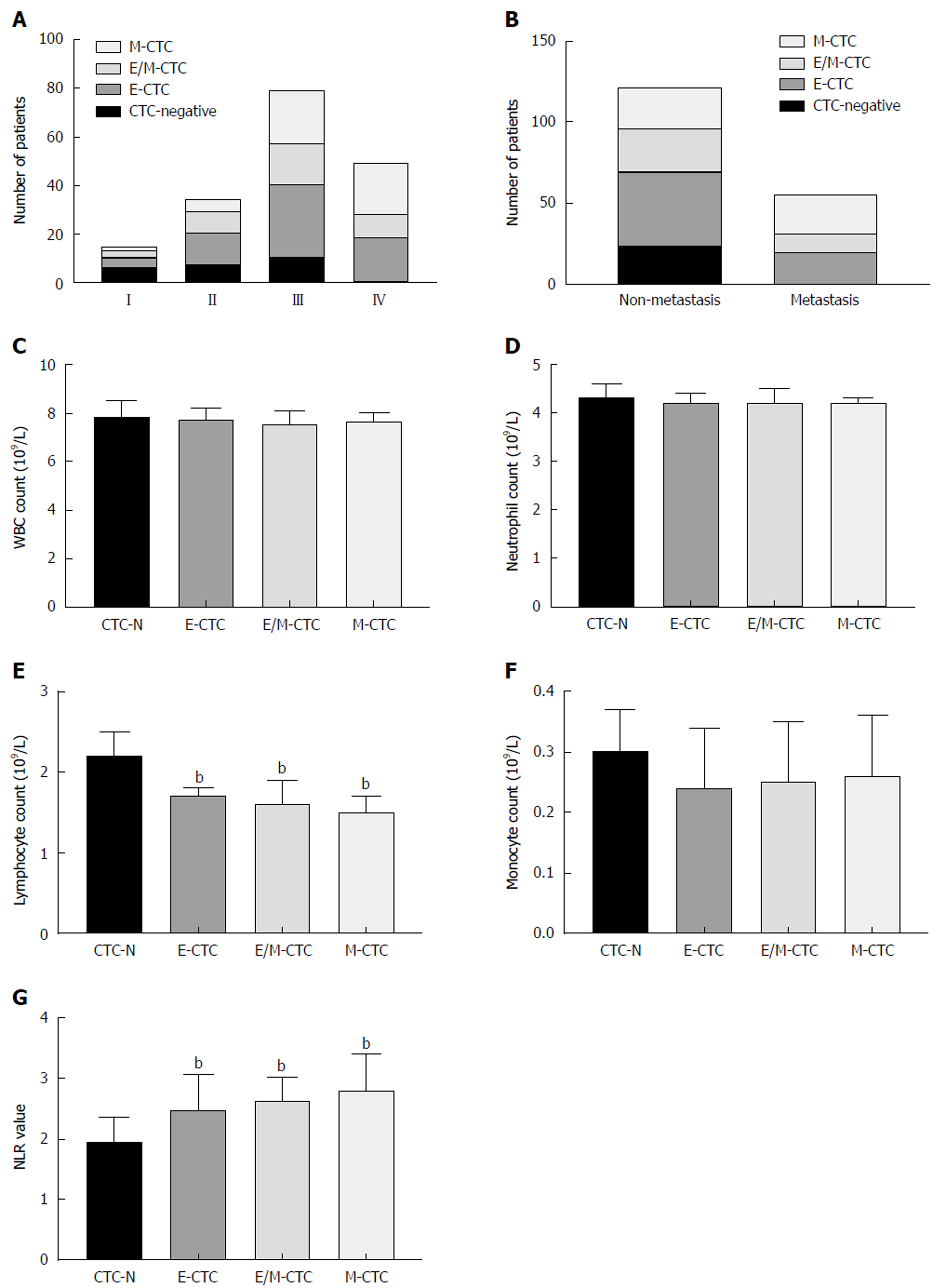
Figure 3 Correlation between circulating tumor cell-epithelial-to-mesenchymal transition subpopulations and clinicopathological characteristics.
A: Distribution of circulating tumor cell (CTC) subpopulations in patients at different AJCC stages of pancreatic ductal adenocarcinoma (PDAC). B: Distribution of CTC subpopulations in patients with or without metastatic disease. C: White blood cell counts in patients without CTCs and positive for epithelial (E)-CTCs, mesenchymal (M)-CTCs, or E/M-CTCs. D: Neutrophil counts in patients without CTCs and positive for E-CTCs, M-CTCs, or E/M-CTCs. E: Lymphocyte counts in patients without CTCs and positive for E-CTCs, M-CTCs, or E/M-CTCs. F: Monocyte counts in patients without CTCs and positive for E-CTCs, M-CTCs, or E/M-CTCs. G: Neutrophil-to-lymphocyte ratio in patients without CTCs and positive for E-CTCs, M-CTCs, or E/M-CTCs. bP < 0.01. CTC: Circulating tumor cells; E-CTC: Epithelial circulating tumor cells; M-CTC: Mesenchymal circulating tumor cells.
- Citation: Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li ZH, Liu YM. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J Gastroenterol 2019; 25(1): 138-150
- URL: https://www.wjgnet.com/1007-9327/full/v25/i1/138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i1.138