Copyright
©The Author(s) 2018.
World J Gastroenterol. Dec 28, 2018; 24(48): 5462-5476
Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5462
Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5462
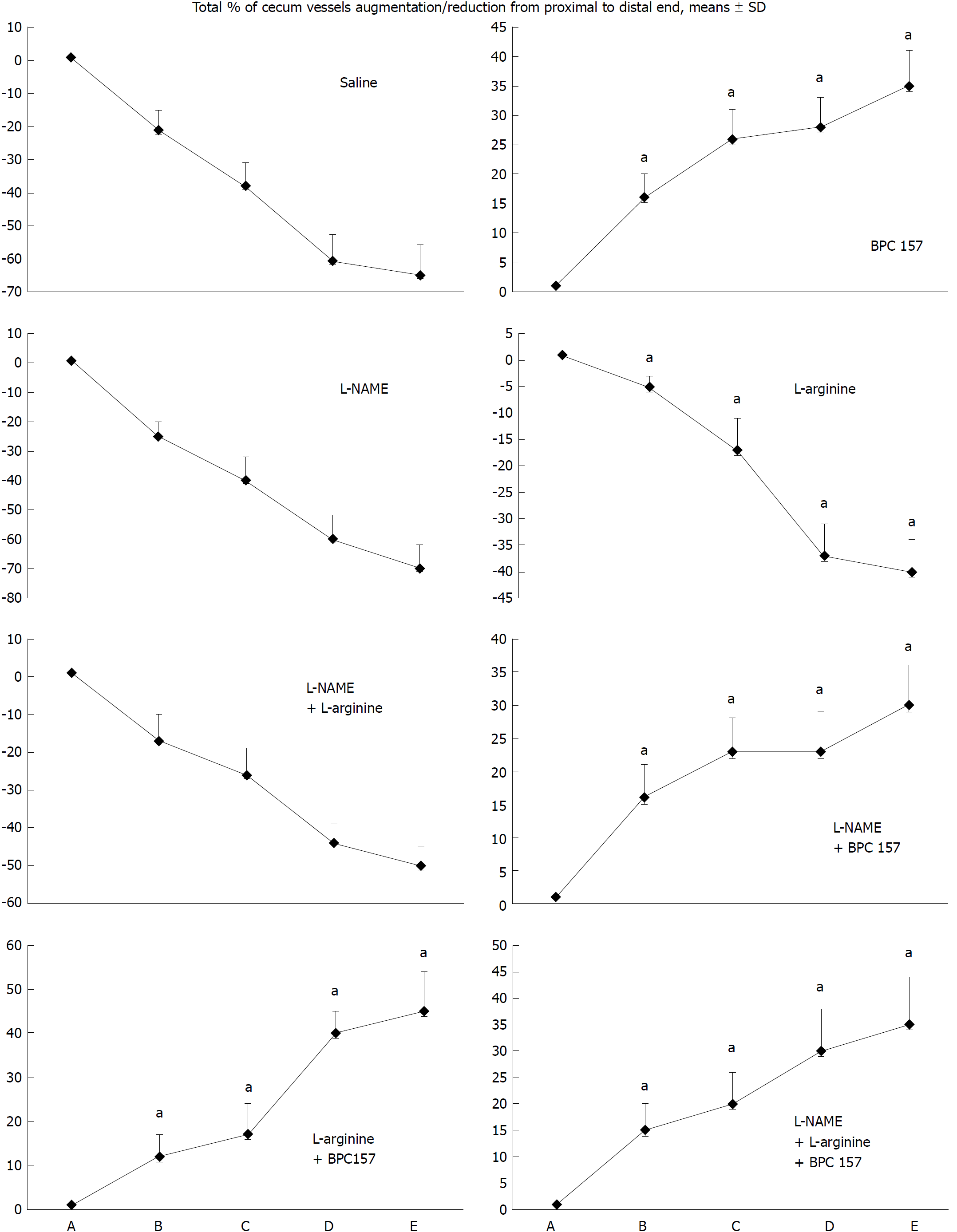
Figure 1 Blood vessels, filled/appearance or cleared out/disappearance (assessed with a USB microscope camera, Veho discovery VMS-004D-400x USB microscope), as [total % of cecum vessels augmentation/reduction from proximal to distal end = [number of blood vessels (10 vessels assessed) /100] x % of augmentation/reducing of each vasa recta (0 as point immediately before therapy) (A)].
A: after perforation (1 min); B: during application (2 min); C: the period after application (2 min); D: the subsequent 5-min period; E: the period until the end of the observation (15 min). At 1 min post-injury, medication (/kg, 10 mL/2 min bath/rat) administered at the perforated (5 mm diameter) cecum lesion and cecum (M-mucosa; S-serosa), including BPC 157 (10 μg), NOS blocker L-NAME (5 mg), NOS substrate L-arginine (100 mg) alone or combined, and saline bath of equal volume (controls). Rats were then left after abdominal closure undisturbed until sacrifice, at day 1 or day 7. aP < 0.05 vs control.
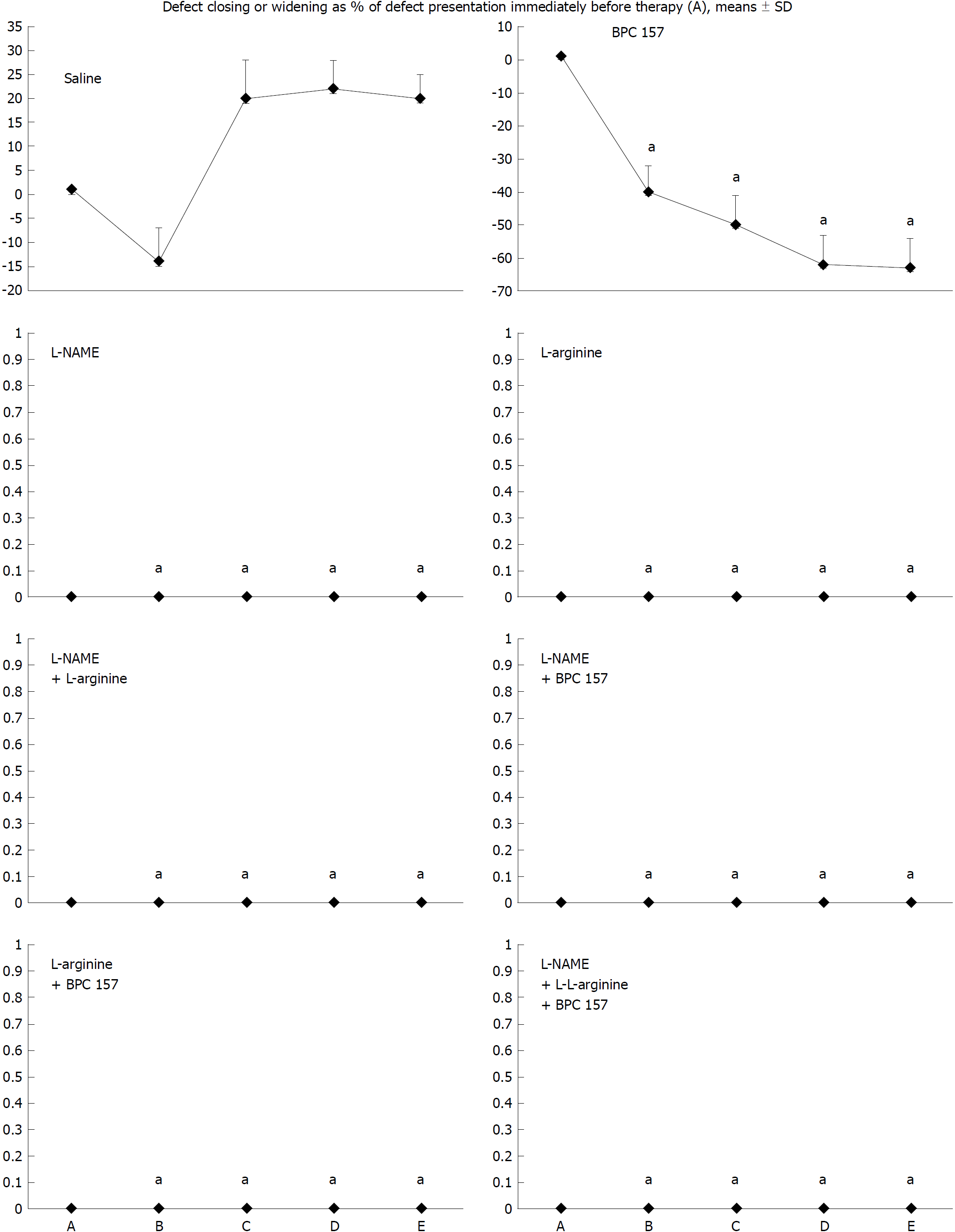
Figure 2 Defect closing or widening [both as % of presentation immediately before therapy (A)]; bleeding time (s); A: after perforation (1 min); B: during application (2 min); C: the period after application (2 min); D: the subsequent 5-min period; E: the period until the end of the observation (15 min).
At 1 min post-injury, administration of medication (/kg, 10 mL/2 min bath/rat) at the perforated (5 mm diameter) cecum lesion and cecum (M-mucosa; S-serosa), including BPC 157 (10 μg), NOS blocker L-NAME (5 mg), NOS substrate L-arginine (100 mg) alone or combined, and a saline bath equal volume (controls). Rats were then left after abdominal closure undisturbed until sacrifice, at day 1 or day 7. aP < 0.05 at least vs control.
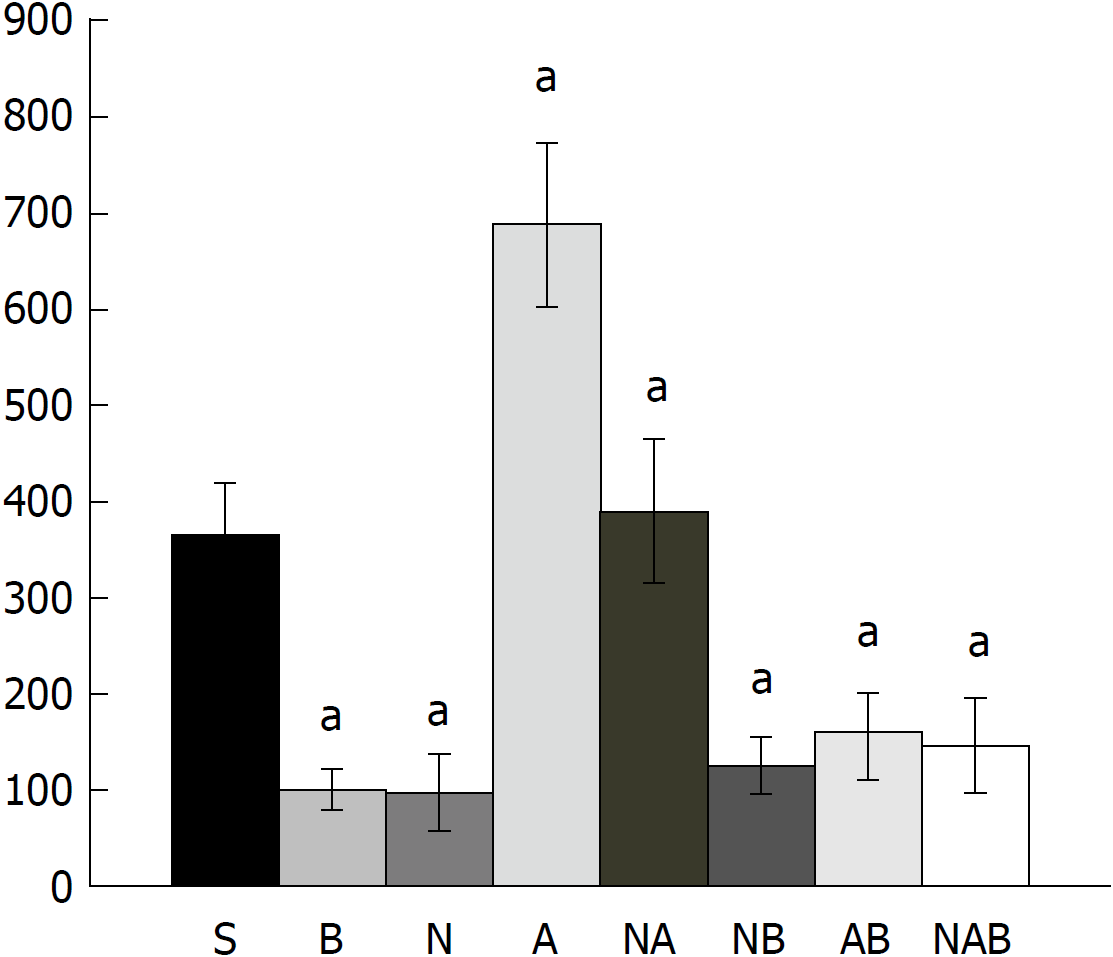
Figure 3 Bleeding time after perforation.
At 1 min post-injury, administration of medication (/kg, 10 mL/2 min bath/rat) at the perforated (5 mm diameter) cecum lesion and cecum includes BPC 157 (10 μg) (B), NOS blocker L-NAME (5 mg) (N), NOS substrate L-arginine (100 mg) (A) alone or combined, and a saline bath of equal volume (controls) (S). Rats were then left after abdominal closure undisturbed until sacrifice, at day 1 or day 7. aP < 0.05 at least vs control (S).
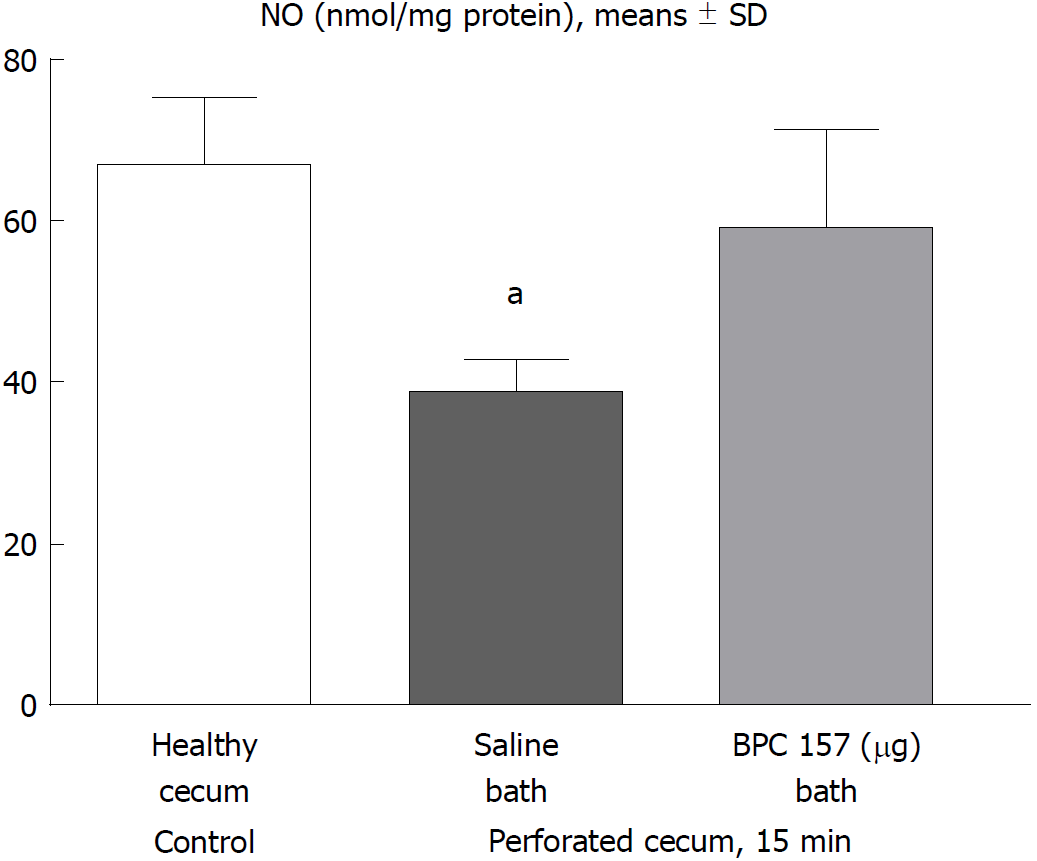
Figure 4 15 min post-injury, we determined nitric oxide in cecum tissue samples using the Griess reaction.
Administration of medication (/kg, 10 mL/2 min bath/rat) at the perforated (5 mm diameter) cecum lesion and cecum was BPC 157 (10 μg) or a saline bath equal volume (controls). Minimum aP < 0.05 vs control.
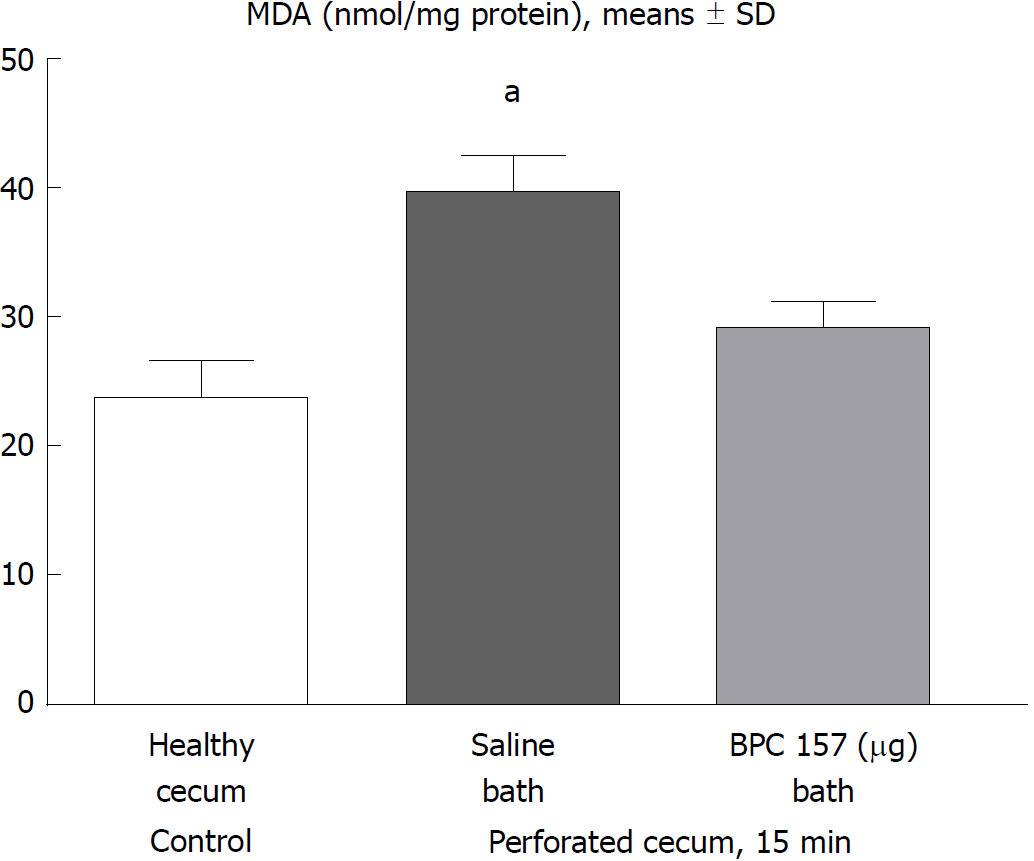
Figure 5 At 15 min post-injury, oxidative stress in tissue samples was assessed by quantifying thiobarbituric acid reactivity as malondialdehyde equivalents.
Administration of medication (/kg, 10 mL/2 min bath/rat) at the perforated (5 mm diameter) cecum lesion and cecum was BPC 157 (10 μg) or a saline bath of equal volume (controls). Minimum aP < 0.05 vs control. MDA: Malondialdehyde equivalents.
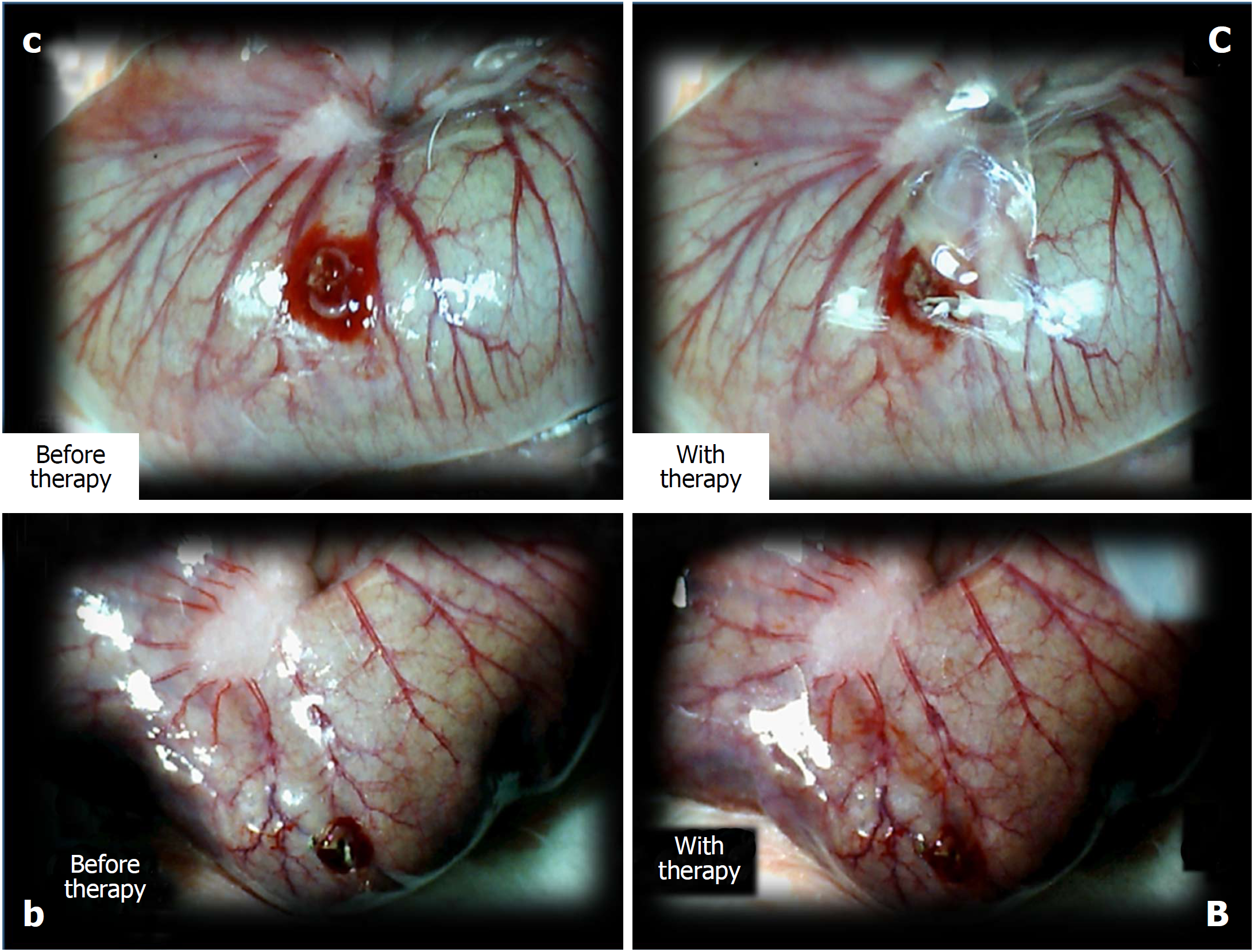
Figure 6 The perforated cecum.
Perforate lesions, before therapy (c, b) (left). Perforate lesions with therapy [control, saline (C), BPC 157 (B)] (right). Illustrative distinctive effect of medication administration (saline bath (right, upper, control (C) vs BPC 157 bath [right, low, BPC 157 (B)]. Regular failing effect of saline bath application on vessels presentation [right, upper, control (C)] vs the immediate recruitment effect of BPC 157 bath administration on the blood vessels presentation toward the perforated injury [right, low, BPC 157 (B)]. Note the initial recovery of blood vessels that appear alongside with the BPC 157 bath, a network raising toward the perforated defect.
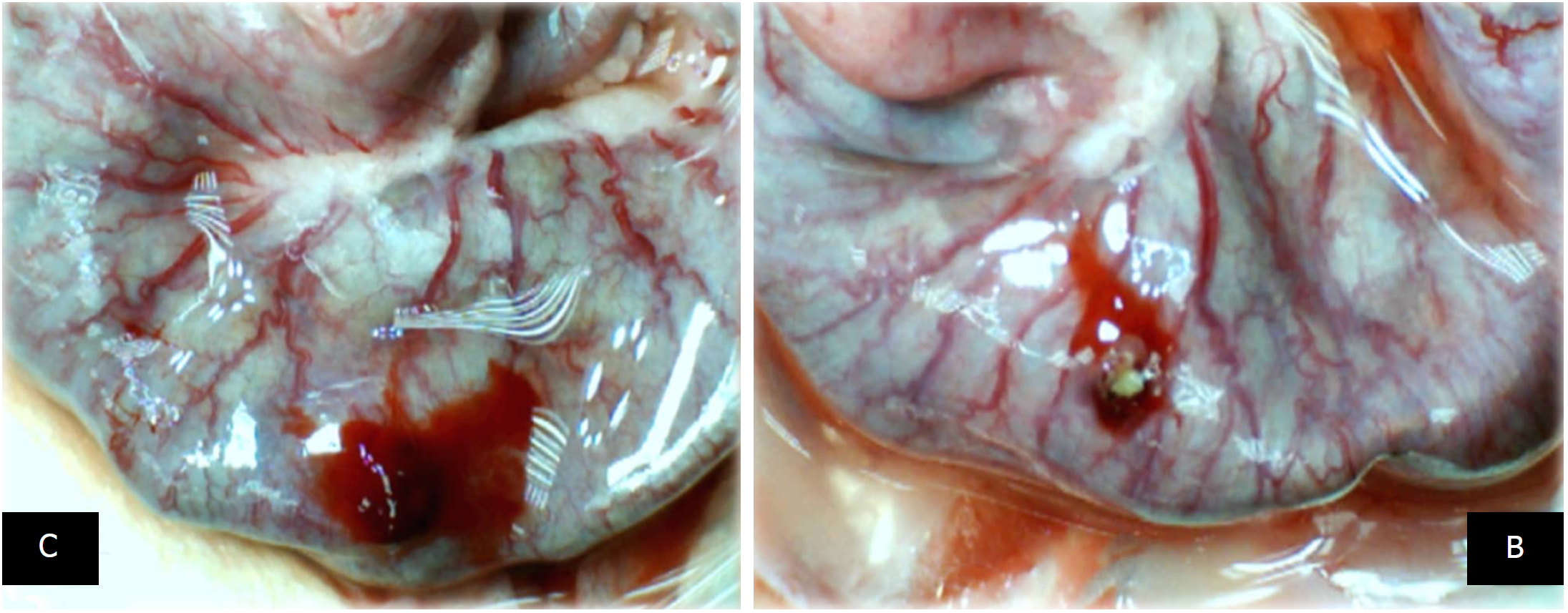
Figure 7 At 10 min, in the perforated cecum, the illustrative effect of medication administration [saline bath (left, control (C)] and BPC 157 bath [right, BPC 157 (B)].
In the damaged cecum, the regular effect of saline bath application on vessels presentation [left, control (C)] vs the immediate effect of BPC 157 bath administration on the blood vessels presentation toward the perforated injury [right, BPC 157 (B)].
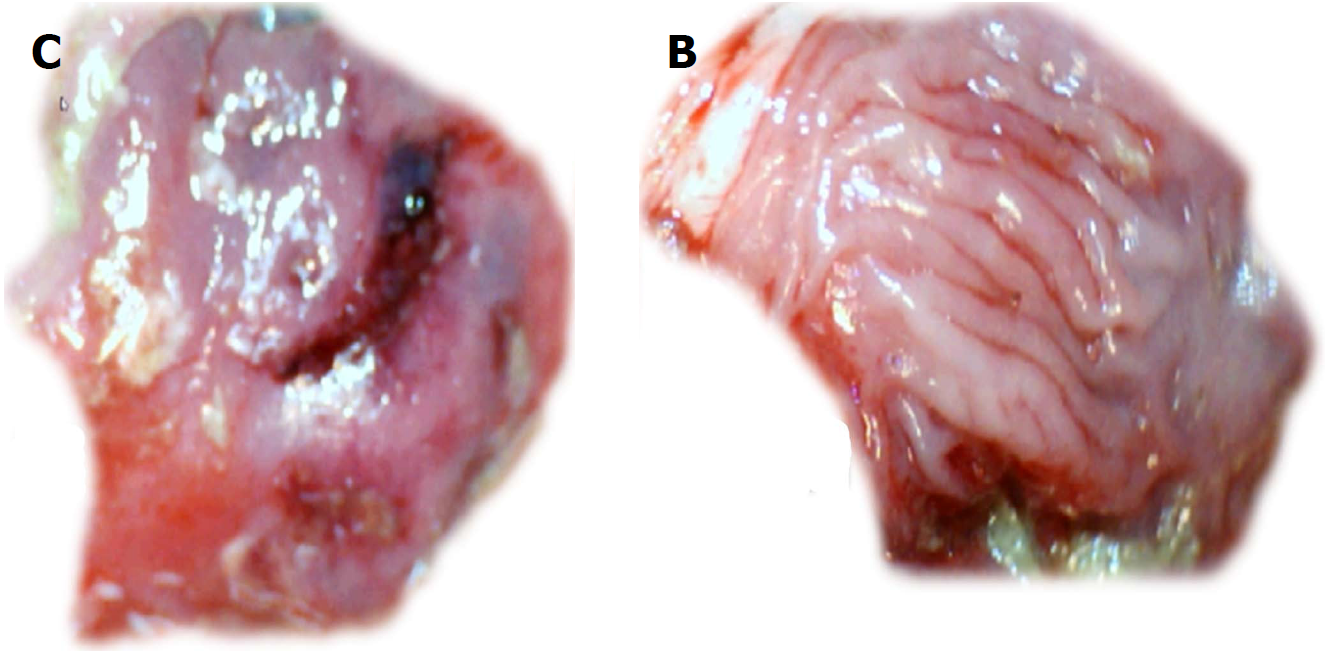
Figure 8 Gross presentation of the area of perforation [left, control (C); right, BPC 157 (B)] and perforated injury [left, control (C)].
Presentation at day 7. Veho discovery VMS-004D-400x USB microscope. Control, 7 d, left, C. Gross lesion shown upon cecum opening before sacrifice. BPC 157, 7 d, mucosa presentation without a grossly visible defect (right, BPC 157 (B)).
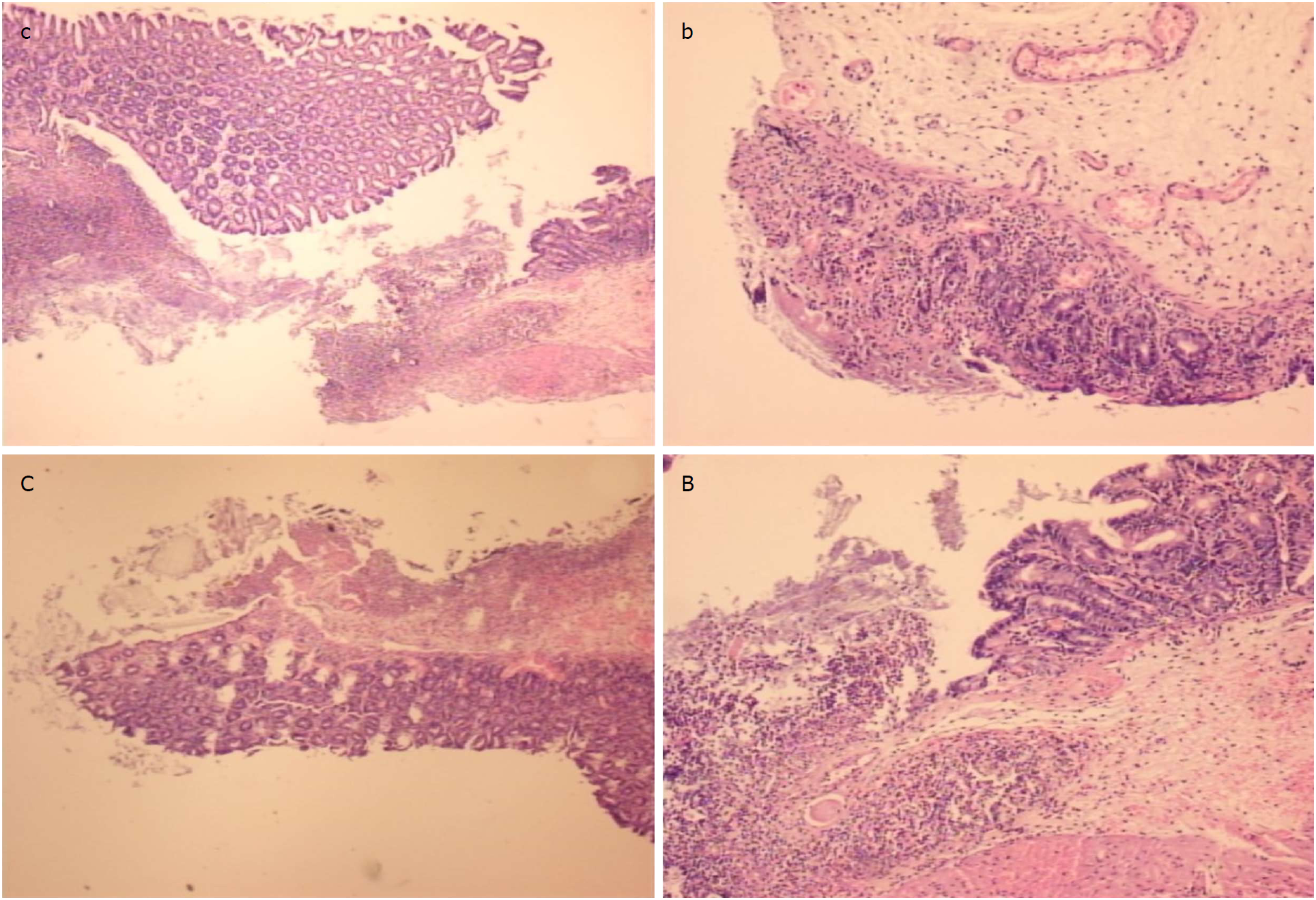
Figure 9 At the first day post-injury, controls (c, C) exhibited intensive edema, loss of glands in the vicinity of the perforation, not possible to obtain a complete defect (due to edema, the tissue is disintegrating), scarce inflammation, and neutrophils [c, HE x 4; C, HE x 10 (control)].
In rats treated with BPC 157 (b, B), we noted much less edema and intensive inflammation (mixed-neutro and mono). Fibrin cloth attached to defect (“holding” the edges) (b, HEx4; B, HEx10 (BPC 157)).
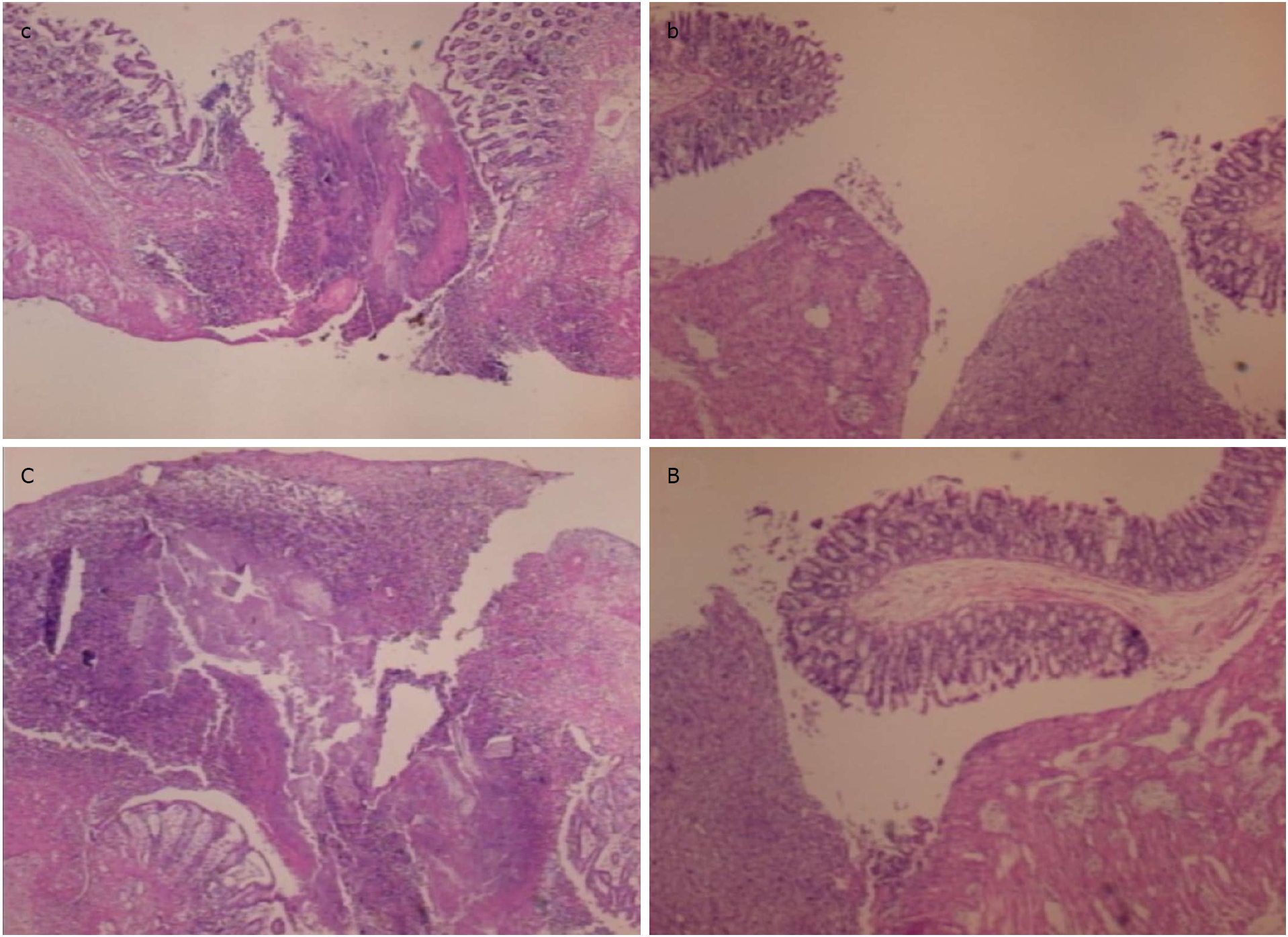
Figure 10 In control animals (c, C), the perforation after 7 d shows a communication between the lumen and peritoneal cavity, partly closed by a loose cloth with many debris and inflammatory cells [c, HE x 4; C, HE x 10 (control)].
In rats treated with BPC 157 (b, B), the defect was sealed with well-formed granulation tissue. The surrounding mucosa in controls was edematous, and the epithelium shows practically no regenerative activity, while in treated animals, the edema was much less pronounced and the surface epithelium started to migrate over the defect [b, HE x 4; B, HE x 10 (BPC 157)].
- Citation: Drmic D, Samara M, Vidovic T, Malekinusic D, Antunovic M, Vrdoljak B, Ruzman J, Milkovic Perisa M, Horvat Pavlov K, Jeyakumar J, Seiwerth S, Sikiric P. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol 2018; 24(48): 5462-5476
- URL: https://www.wjgnet.com/1007-9327/full/v24/i48/5462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i48.5462