Copyright
©The Author(s) 2018.
World J Gastroenterol. Oct 7, 2018; 24(37): 4263-4271
Published online Oct 7, 2018. doi: 10.3748/wjg.v24.i37.4263
Published online Oct 7, 2018. doi: 10.3748/wjg.v24.i37.4263
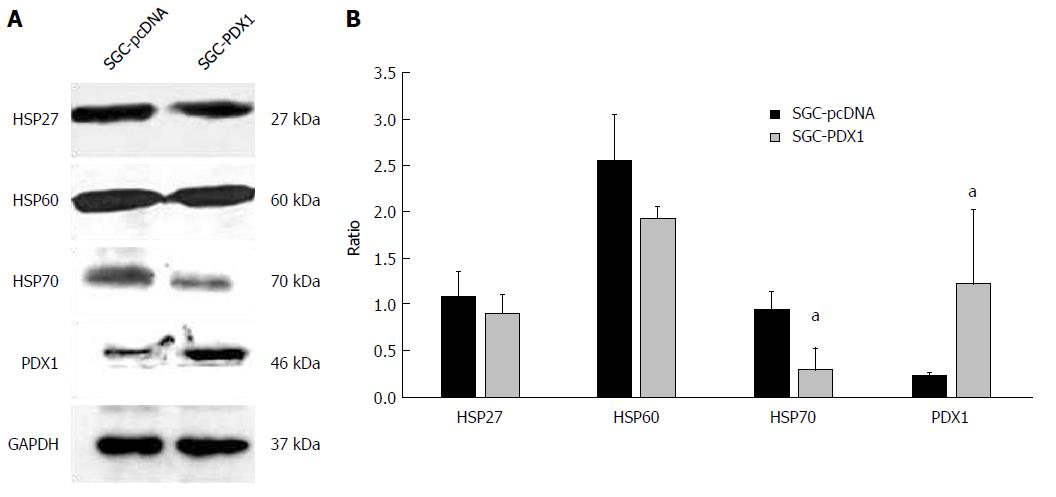
Figure 1 Pancreatic-duodenal homeobox-1 overexpression regulated the expression of heat shock proteins in gastric cancer SGC7901 cells.
A: Immunoblotting analysis confirmed the enhanced expression of PDX1 protein after PDX1-pcDNA transfection in SGC7901 cells. It also revealed that PDX1 overexpression could downregulate the expression of HSP27, HSP60, and HSP70 proteins in SGC-PDX1 cells; B: Quantitative analysis of the results of immunoblotting indicated that HSP70 protein was significantly decreased in SGC-PDX1 cells. aP < 0.05.
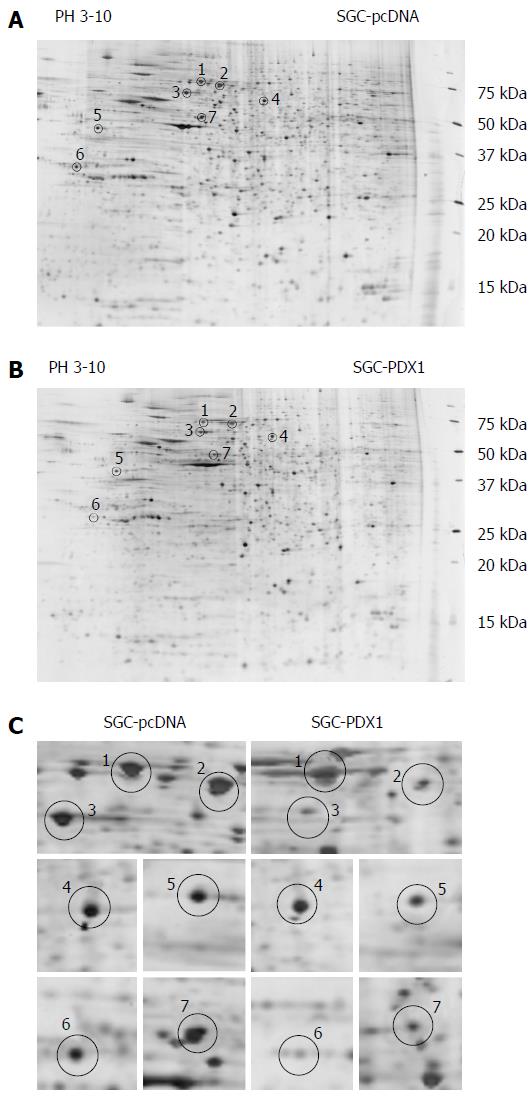
Figure 2 Two-dimensional electrophoresis-gel images of proteins from SGC7901 with pcDNA3.
1(+)-pancreatic-duodenal homeobox-1 (PDX1) vector and SGC7901-pcDNA cells. A and B: Representative 2DE-gel images of SGC-PDX1 and SGC-pcDNA groups. The differentially expressed proteins are indicated by circles; C: Close-up images of the differential protein spots between SGC-PDX1 and SGC-pcDNA groups. 2DE: 2-dimensional electrophoresis.
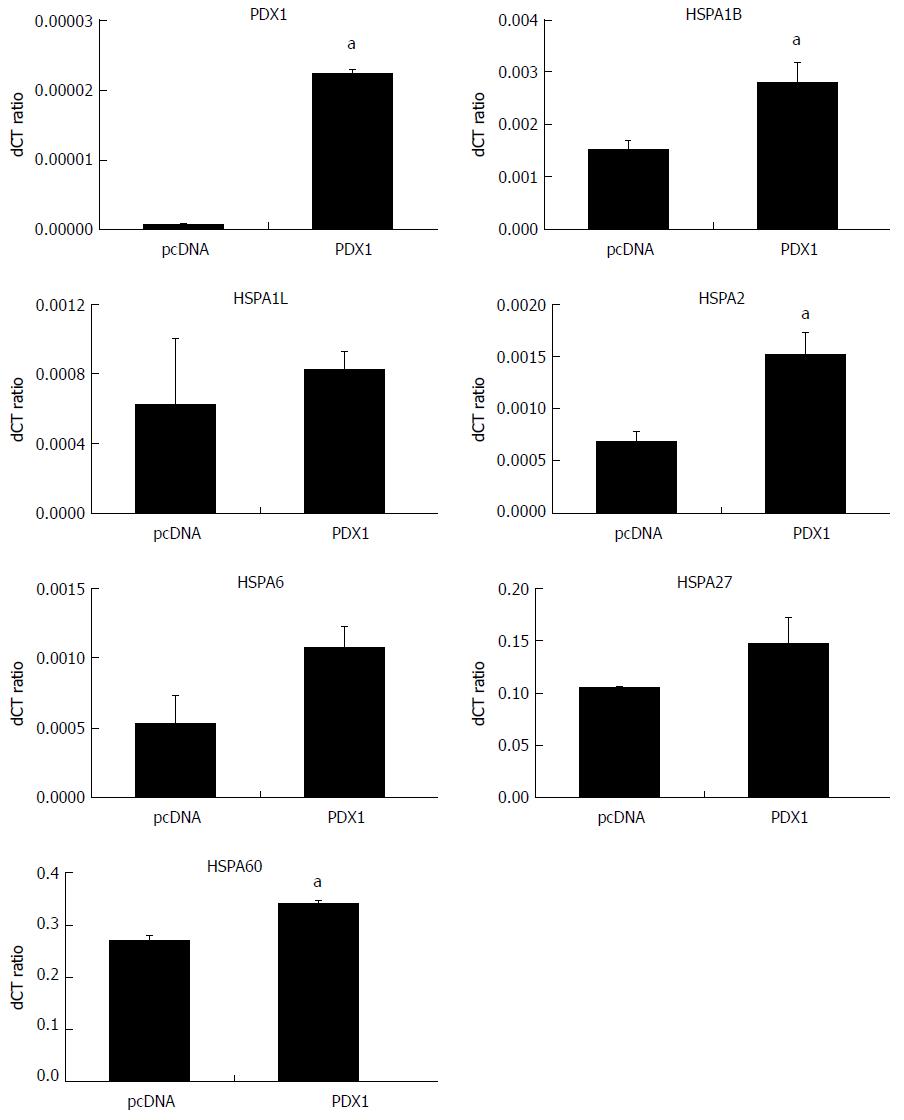
Figure 3 Comparisons of mRNA levels of the heat shock proteins in SGC7901 with pcDNA3.
1(+)-pancreatic-duodenal homeobox-1 (PDX1) vector and SGC7901-pcDNA cells. The mRNA expressions of HSPs in SGC-PDX1 and SGC-pcDNA cells were compared by qRT-PCR. The relative mRNA levels of two of HSP70 isoforms (HSPA1B and HSPA2) and HSP60 were significantly increased in the SGC-PDX1 group, while no significant differences were identified for the other HSP genes. (aP < 0.05). HSPs: Heat shock proteins.
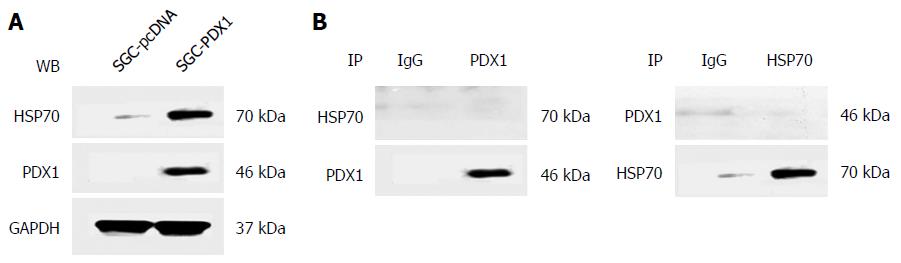
Figure 4 Results of co-immunoprecipitation analysis showing no direct interaction of pancreatic-duodenal homeobox-1 (PDX1) and HSP70 in SGC7901 with pcDNA3.
1(+)-PDX1 cells. A: Both PDX1 and HSP70 proteins were detected in the whole cell lysate proteins of co-transfected SGC7901 cells, ensuring the input of PDX1 and HSP70 proteins in the co-immunoprecipitation experiment; B: The immunoprecipitated proteins obtained by precipitating antibodies against PDX1 or HSP70 were immunoblotted by HSP70 or PDX1, respectively. No direct interactions between PDX1 and HSP70 were detected.
- Citation: Ma J, Wang BB, Ma XY, Deng WP, Xu LS, Sha WH. Potential involvement of heat shock proteins in pancreatic-duodenal homeobox-1-mediated effects on the genesis of gastric cancer: A 2D gel-based proteomic study. World J Gastroenterol 2018; 24(37): 4263-4271
- URL: https://www.wjgnet.com/1007-9327/full/v24/i37/4263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i37.4263