Copyright
©The Author(s) 2018.
World J Gastroenterol. Jun 28, 2018; 24(24): 2567-2581
Published online Jun 28, 2018. doi: 10.3748/wjg.v24.i24.2567
Published online Jun 28, 2018. doi: 10.3748/wjg.v24.i24.2567
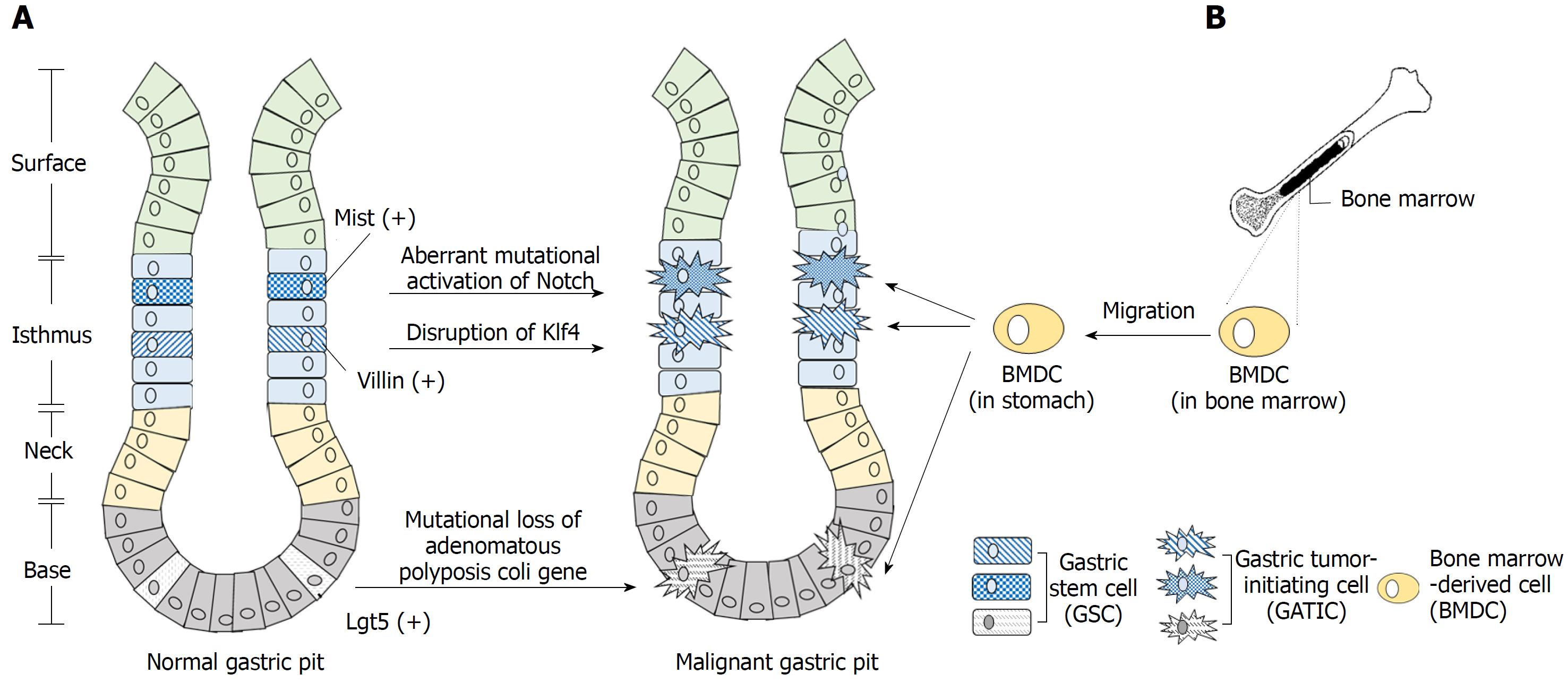
Figure 1 Origination of gastric tumor-initiating cells.
A: Evidence suggests that the gastric stem cell (GSC) is one major origin of the gastric tumor-initiating cell (GATIC). GSCs exist in the isthmus and bottom of the gastric pit. Certain genetic, epigenetic, and/or environmental factors potentially transform these GSCs into malignant GATICs. For instance, Mist(+) and Villin(+) GSCs in the isthmus and Lgr5(+) GSCs in the bottom could act as the origins of GATICs through multiple signaling pathways; B: GATICs are also presumed to originate partially from bone marrow-derived cells (BMDCs). The recruitment of BMDCs to stomach by chemokines and other factors is in parallel with the multi-step progression of gastric cancer (GC), which lays the basis for the presumption that BMDCs undergo the malignant transformation into GATICs and promote GC development, the underlying mechanism of which requires further investigation.
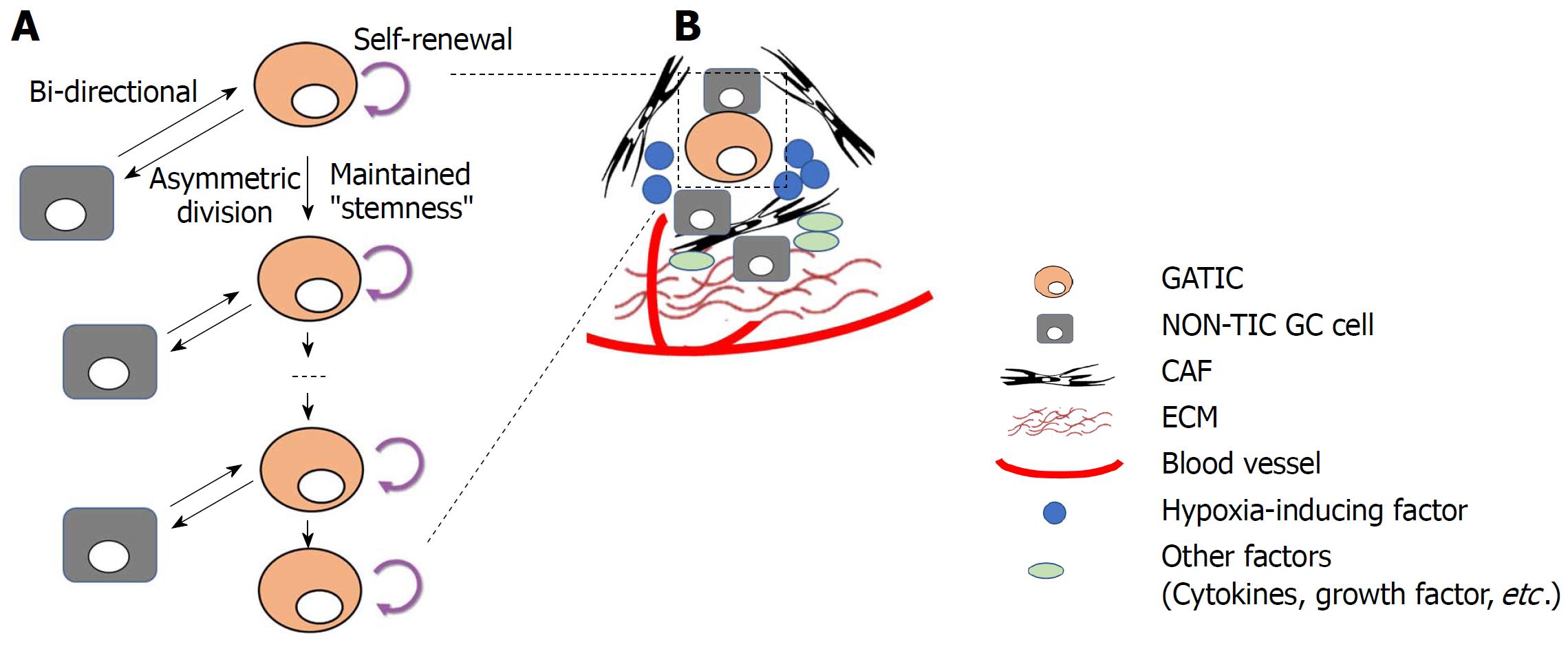
Figure 2 Plasticity of gastric tumor-initiating cells.
A: Gastric tumor-initiating cells (GATICs) give rise to both daughter GATICs and non-TIC gastric cancer (GC) cells (asymmetric division) while maintain their self-renewal capacity and “stemness”. Notably, recent studies demonstrate that differentiated non-TIC GC cells could undergo dedifferentiation and re-acquire the properties (or status) of GATICs. Thus, the bi-directional transition between TIC and non-TIC indicates the plasticity of GATICs, which is regulated by both genetic/epigenetic alterations and tumor microenvironmental factors; B: GATICs reside in the tumor-microenvironment, which consists of cancer cells (GATICs and non-TIC GC cells) as well as non-cancerous cells, such as cancer-associated fibroblast, extracellular matrix, blood supply, hypoxia (hypoxia-inducing factor), and other secreted factors, such as cytokines, growth factors. GATICs interact with these factors within the TIC niche, which exerts regulatory influence on the plasticity of GATICs through various signaling pathways.
- Citation: Gao JP, Xu W, Liu WT, Yan M, Zhu ZG. Tumor heterogeneity of gastric cancer: From the perspective of tumor-initiating cell. World J Gastroenterol 2018; 24(24): 2567-2581
- URL: https://www.wjgnet.com/1007-9327/full/v24/i24/2567.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i24.2567