Copyright
©The Author(s) 2018.
World J Gastroenterol. Apr 7, 2018; 24(13): 1478-1485
Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1478
Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1478
Figure 1 Treatment regimen of sofosbuvir and ribavirin.
SOF: Sofosbuvir; RBV: Ribavirin; EOT: End of treatment.
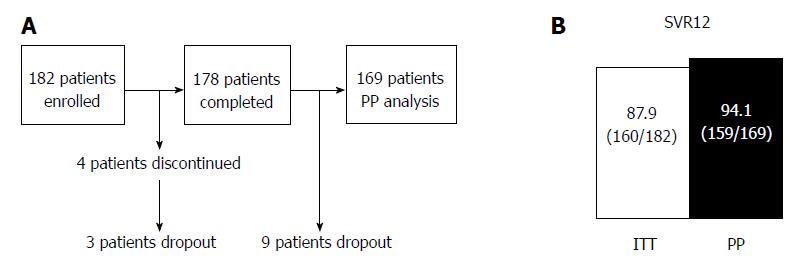
Figure 2 Flow sheet of this study (A) and virological response rates for combination therapy with sofosbuvir and ribavirin (B).
The rates of sustained virological response at 12 wk after the end of treatment are shown for intention to treat and per protocol analyses. PP: Per protocol; ITT: Intention to treat; SVR12: Sustained virological response at 12 wk after the end of treatment.
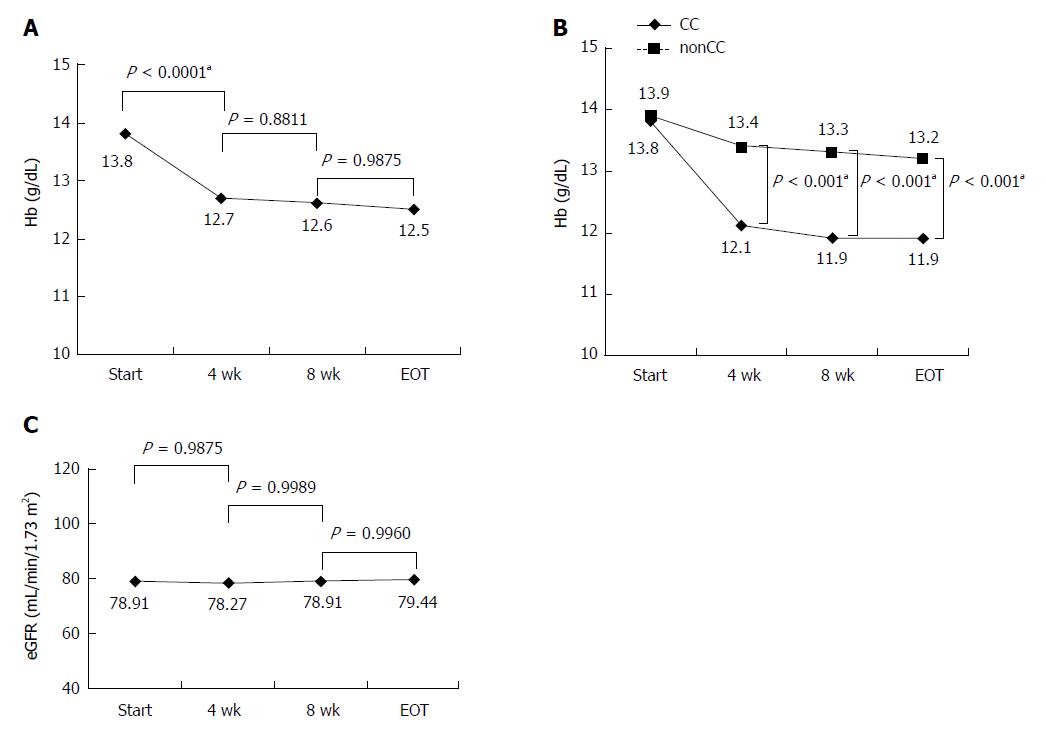
Figure 3 Hemoglobin levels in all patients during combination therapy with sofosbuvir and ribavirin (A), hemoglobin levels in 175 patients categorized by inosine triphosphate pyrophosphatase single nucleotide polymorphism rs1127354 (CC or non CC) (B), and estimated glomerular filtration rate levels in all patients during sofosbuvir/ribavirin therapy (C).
Hb: Hemoglobin; EOT: End of treatment; eGFR: Estimated glomerular filtration rate. aP < 0.05, significant difference.
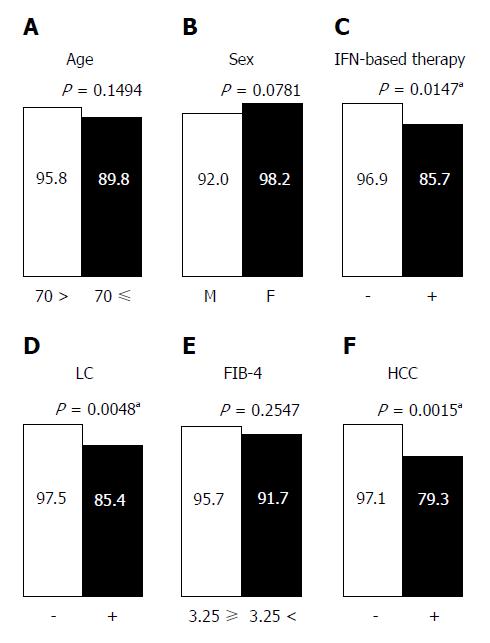
Figure 4 Virological response in patients with sofosbuvir and ribavirin (SOF/RBV) combination therapy categorized by patient characteristics.
A: Age (< 70 or ≥ 70 yr); B: Sex (male or female); C: History of interferon-based therapy (− or +); D: Liver cirrhosis (− or +); E: Fibrosis-4 index (≤ 3.25 or > 3.25); F: History of hepatocellular carcinoma (− or +). aP < 0.05: Significant difference. M: Male; F: Female; IFN: Interferon; LC: Liver cirrhosis; FIB: Fibrosis; HCC: Hepatocellular carcinoma.
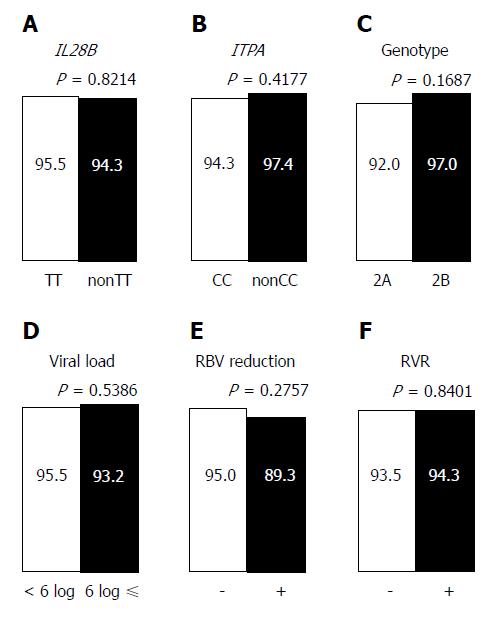
Figure 5 Virological response in patients with sofosbuvir and ribavirin combination therapy categorized by single nucleotide polymorphisms related to anti-hepatitis C virus therapy, pretreatment viral status, ribavirin dose, and rapid virological response.
A: IL28B single nucleotide polymorphisms rs8099917 (TT or non TT); B: ITPA single nucleotide polymorphisms rs1127354 (CC or non CC); C: Hepatitis C virus genotype (2A or 2B); D: Pretreatment viral load (< 6 logIU/mL or ≥ 6 logIU/mL); E: Ribavirin dose reduction (− or +); F: Rapid virological response (− or +). aP < 0.05: Significant difference. IL28B: Interleukin-28B; ITPA: Inosine triphosphate pyrophosphatase; RBV: Ribavirin; RVR: Rapid virological response.
- Citation: Yada M, Miyazaki M, Tanaka K, Masumoto A, Motomura K. Hepatocellular carcinoma or interferon-based therapy history attenuates sofosbuvir/ribavirin for Japanese genotype 2 hepatitis C virus. World J Gastroenterol 2018; 24(13): 1478-1485
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1478