Copyright
©The Author(s) 2018.
World J Gastroenterol. Mar 21, 2018; 24(11): 1181-1195
Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1181
Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1181
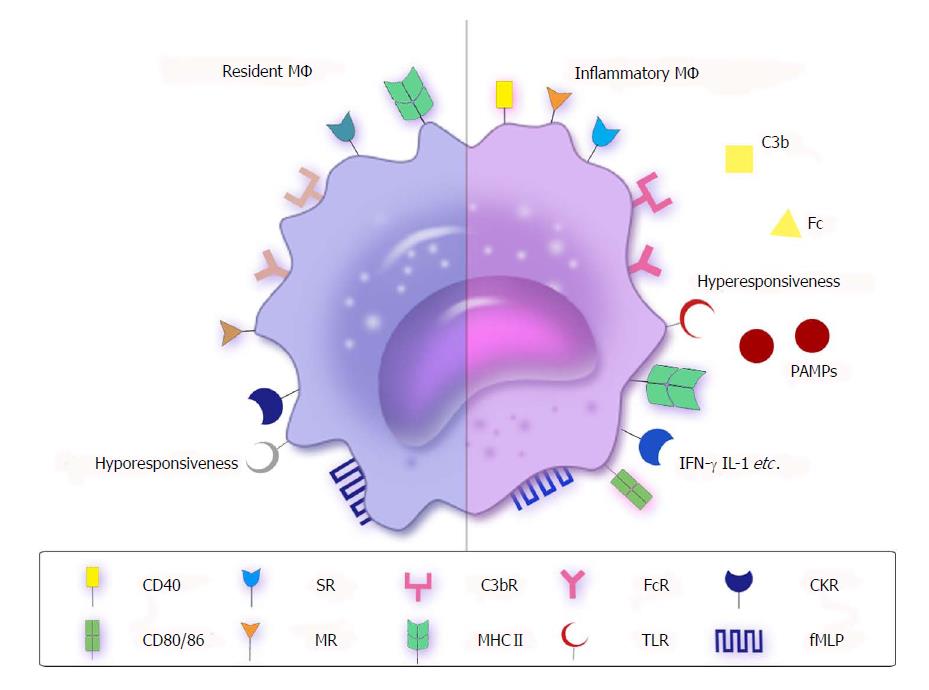
Figure 1 Receptors or molecules of resident and inflammatory macrophages.
MΦ express opsonic (FcR and C3bR) or nonopsonic receptors, such as CKRs, MRs, SRs, fMLP and TLRs, as well as express high levels of MHC II. However, there are some differences between resident MΦ and inflammatory MΦ. Resident MΦ (left side) do not express high levels of costimulatory molecules such as CD40, CD80 and CD86, and present hyporesponsiveness to TLRs to suppress inflammation. However, inflammatory MΦ (right side) show the opposite trend. The PAMPs lead to inflammation by connecting with hyperresponsive TLRs. CKR: Cytokine receptor; fMLP: Formyl-methionine-leucyl-phenylalanine; MR: Mannose receptor; MΦ: Macrophages; PAMP: Pathogen-associated molecular pattern; SR: Scavenger receptor; TLR: Toll-like receptor.
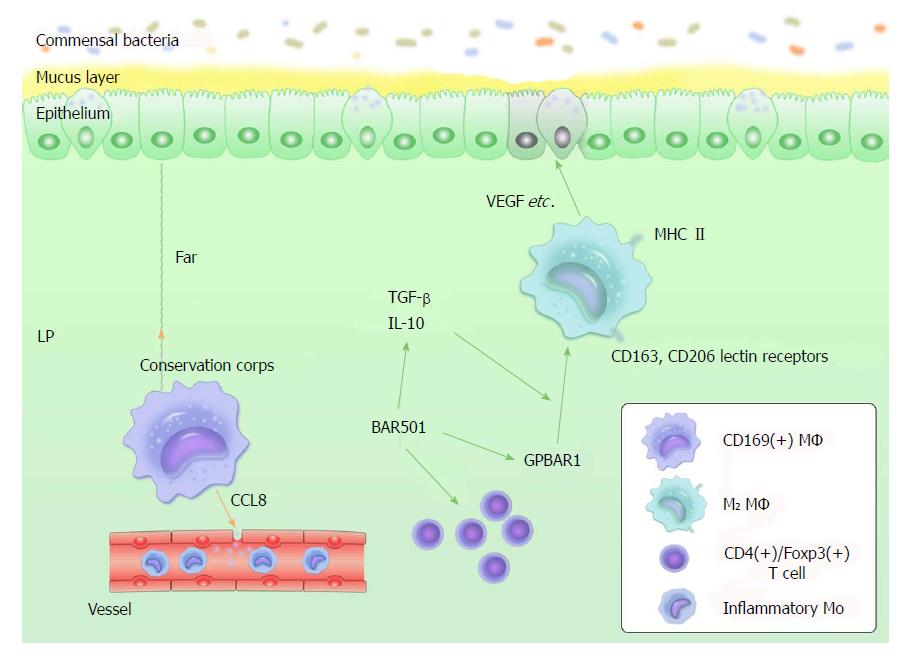
Figure 2 Current views about intestinal macrophages.
(1) LP-resident CD169+ MΦ reside at the bottom-end of the LP microenvironment, far away from the epithelium-LP border. CD169+ MΦ recruit inflammatory monocytes by producing CCL8. CD109+ MΦ can be considered as a "conservation corps" in the intestine because they likely respond to the collapse of frontline defense; (2) M2 MΦ are MHCII+, producing IL-10 and expressing CD163, CD206 and lectin receptors. They do not produce proinflammatory mediators with signals of stimulation. In addition, they produce tissue-repairing factors, such as VEGF, actin and metalloproteinases; and (3) GPBAR1 is essential to maintain intestinal immune homeostasis by regulating M1/M2 MΦ. BAR501 is a small-molecule stimulus for GPBAR1. It contributes to this regulative process, depending on the production control of IL-10. Exposure to BAR501 leads to increased expression of IL-10, TGF-β mRNA and the percentage of CD4+/Foxp3+ cells. IL: Interleukin; LP: Lamina propria; MΦ: Macrophages; TGF: Tumor growth factor; VEGF: Vascular endothelial growth factor.
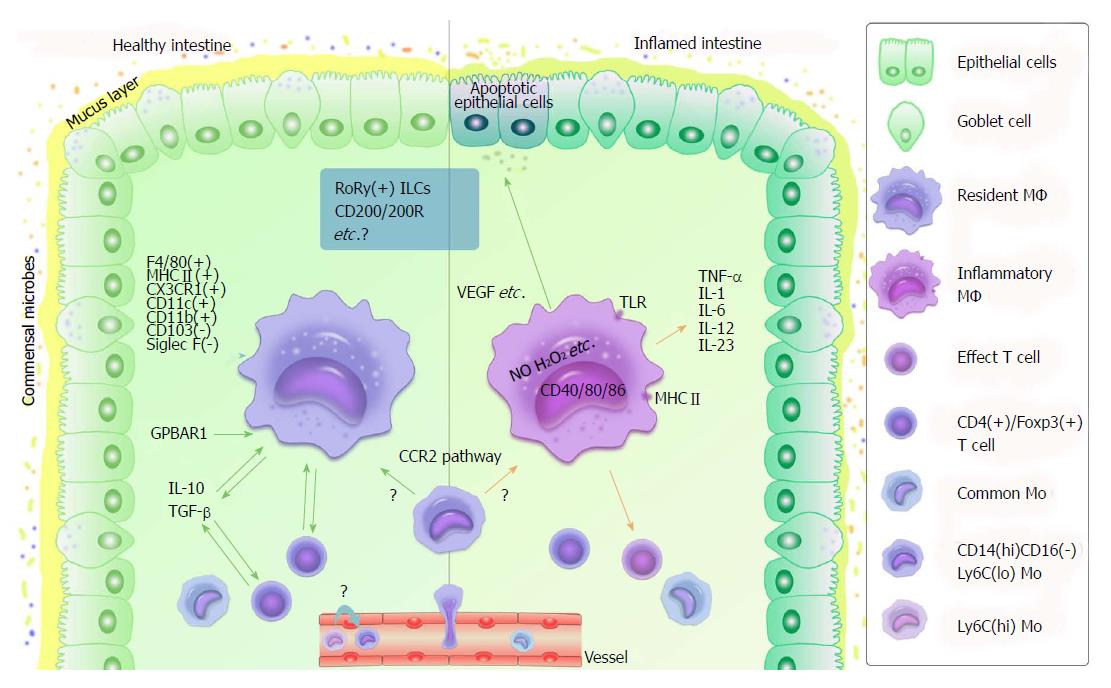
Figure 3 Functional role of macrophages in healthy or inflamed intestine.
MΦ differentiate from blood Mo. Ly6clo Mo are proposed to be the precursors of resident MΦ. CD14hiCD16- Mo turn into resident or inflammatory MΦ according to different circumstances via the CCR2 pathway. In healthy intestine (left side), resident MΦ are F4/80hi, class II MHChi CX3CR1hi, CD11c+, CD103- and Siglec F-. They do not express high levels of costimulatory molecules such as CD40, CD80 and CD86. Their connections with CD4+/Foxp3+ T cells, IL-10 and TGF-β are helpful to maintain intestinal homeostasis (green arrows). GPBAR1 is essential to maintain intestinal immune homeostasis by regulating M1/M2 MΦ. In inflamed intestine (right side), Mo change into inflammatory MΦ, which produce TNF-β, IL-1, IL-6, IL-12, and IL-23, and activate effective T cells with several specific receptors, such as TLR, as well as induce respiratory burst (e.g., NO and H2O2 production), leading to inflammation (orange arrows). In addition, M2 MΦ produce tissue-repairing factors such as VEGF, which shows a positive effect in individuals during inflammation (green arrow). Regarding MΦ and intestinal immunity, many details remain unclear - for instance, the functions of RoRy+ ILCs and CD200/200R (in blue rectangle) as well as that of Ly6Chi/lo Mo. IL: Interleukin; ILC: Innate lymphoid cell; Mo: Monocytes; MΦ: Macrophages; NO: Nitric oxide; TGF: Tumor growth factor; TLR: Toll-like receptor; TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor.
- Citation: Liu YH, Ding Y, Gao CC, Li LS, Wang YX, Xu JD. Functional macrophages and gastrointestinal disorders. World J Gastroenterol 2018; 24(11): 1181-1195
- URL: https://www.wjgnet.com/1007-9327/full/v24/i11/1181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i11.1181