Copyright
©The Author(s) 2017.
World J Gastroenterol. Feb 28, 2017; 23(8): 1489-1496
Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1489
Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1489
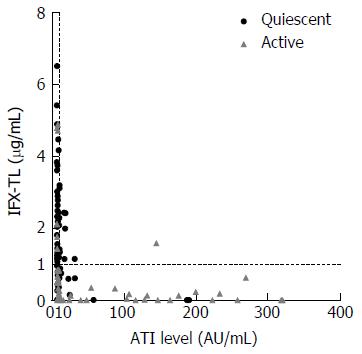
Figure 1 Scatter diagram of the study patients.
Of these 138 subjects, 84 (60.9%) had quiescent disease and 54 (39.1%) had active disease. The overall median infliximab trough level (IFX-TL) value was 0.941 μg/mL. In total, 91 patients were antibodies to infliximab (ATI) negative (65.9%) and 47 patients were ATI positive (34.1%), with an overall median ATI value of 8.846 (IQR 7.719-16.727) AU/mL.
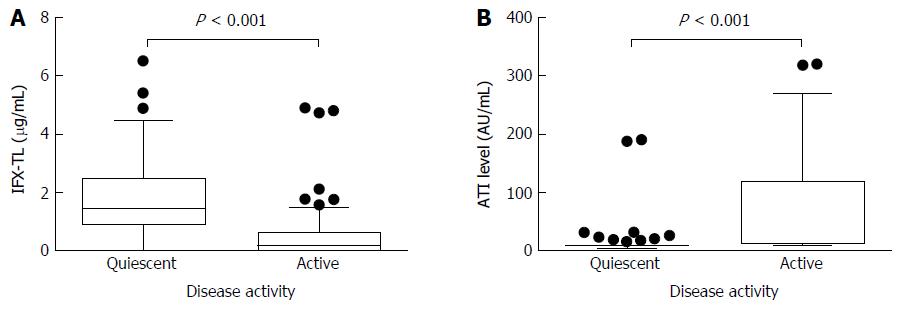
Figure 2 Comparisons of the infliximab trough levels and antibody to infliximab levels between patients with quiescent or active disease.
The median infliximab trough levels (IFX-TLs) were 1.423 μg/mL (IQR 0.877-2.483) and 0.163 μg/mL (IQR 0.002-0.636), respectively (P < 0.001) (A) and the median antibodies to infliximab (ATIs) levels were 8.064 AU/mL (IQR 6.929-9.908) and 11.209 AU/mL (IQR 8.008-118.835), respectively (P < 0.001) (B) in patients with quiescent and active disease.
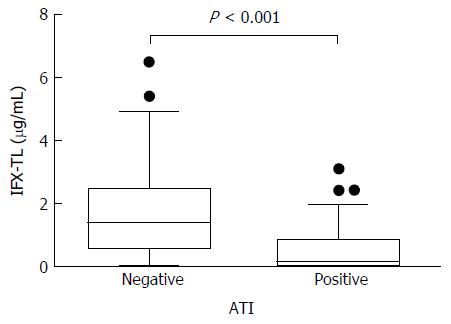
Figure 3 Comparison of the infliximab trough levels between patients with/without antibody to infliximab.
In the ATI-negative and -positive groups, the median infliximab trough levels (IFX-TLs) were 1.415 μg/mL (IQR 0.570-2.495) and 0.141 μg/mL (IQR 0.002-0.869), respectively (P < 0.001). ATI: Antibodies to infliximab.
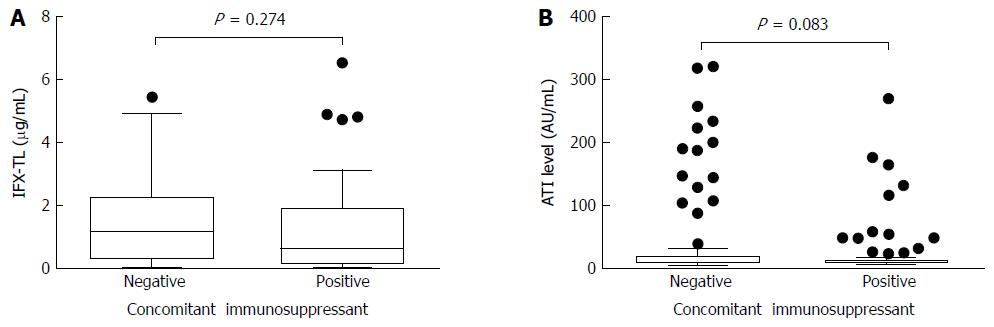
Figure 4 Comparisons of infliximab trough levels and antibody to infliximab levels in patients with/without immunomodulators.
There were no differences in the median infliximab trough level (IFX-TL) (0.632 μg/mL and 1.150 μg/mL, respectively; P = 0.274) (A) and median antibodies to infliximab (ATIs) (8.655 AU/mL and 9.017 AU/mL, respectively; P = 0.083) (B) levels between the 2 groups according to the use of concomitant immunomodulators.
- Citation: Oh EH, Ko DH, Seo H, Chang K, Kim GU, Song EM, Seo M, Lee HS, Hwang SW, Yang DH, Ye BD, Byeon JS, Myung SJ, Yang SK, Park SH. Clinical correlations of infliximab trough levels and antibodies to infliximab in South Korean patients with Crohn’s disease. World J Gastroenterol 2017; 23(8): 1489-1496
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1489.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1489