Copyright
©The Author(s) 2017.
World J Gastroenterol. Dec 28, 2017; 23(48): 8443-8451
Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8443
Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8443
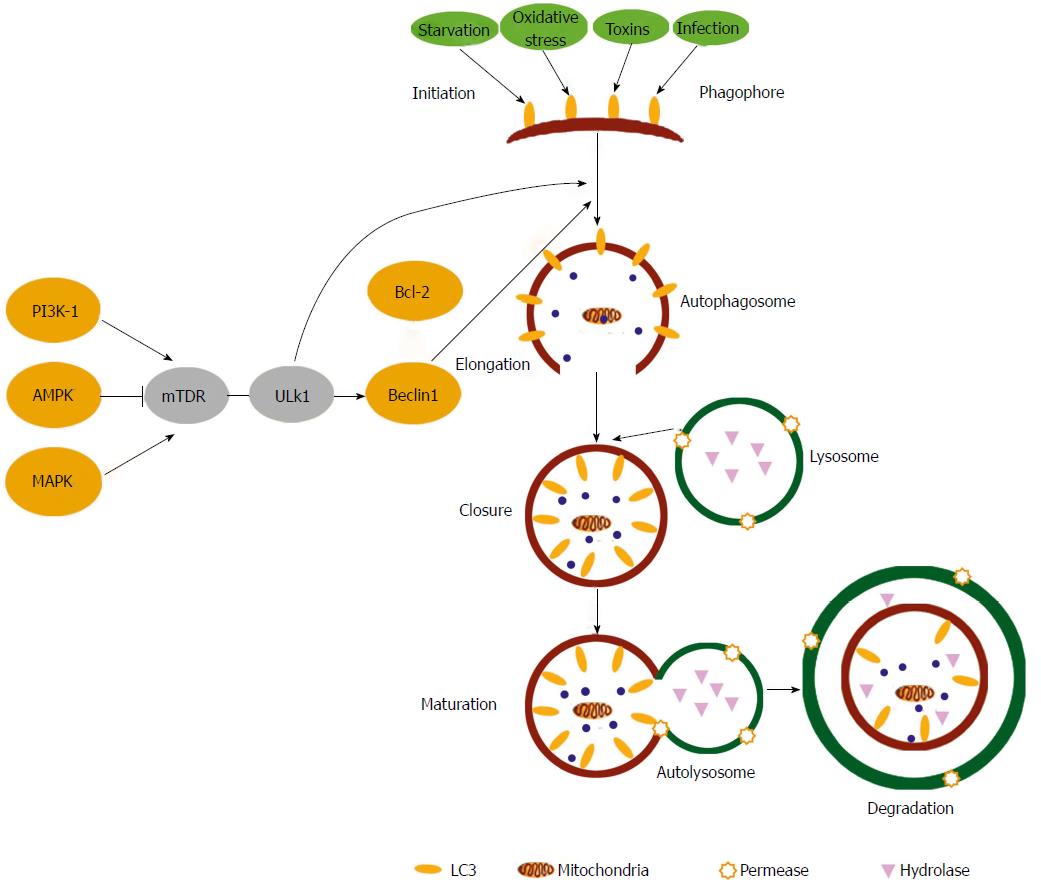
Figure 1 Autophagy signalling pathways.
Upstream autophagy activation is regulated by the integration of signalling from a number of pathways including AMPK, PI-3K and the Mitogen-Activated Protein Kinases. Phagophore initiation is directly regulated by the serine/threonine protein kinases ULK1 that forms a complex with Beclin 1. Upon initiation, cytoplasmic constituents are enclosed in a double membraned isolation structure known as an autophagosome that is elongated mainly through the action of two ubiquitin-like conjugation systems. Autophagosomes fuse with lysosomes to form autophagolysosomes, where breakdown of the vesicle contents/cargo takes place along with the autophagosome inner membrane. Autophagy can be activated by many stimuli including starvation, toxins, oxidative stress and infections. (Taken from Shan NN et al Targeting autophagy as a potential therapeutic approach for immune thrombocytopenia therapy. Crit Rev Oncol Hematol 2016; 100: 11-15. DOI: 10.1016/j.critrevonc.2016.01.011).
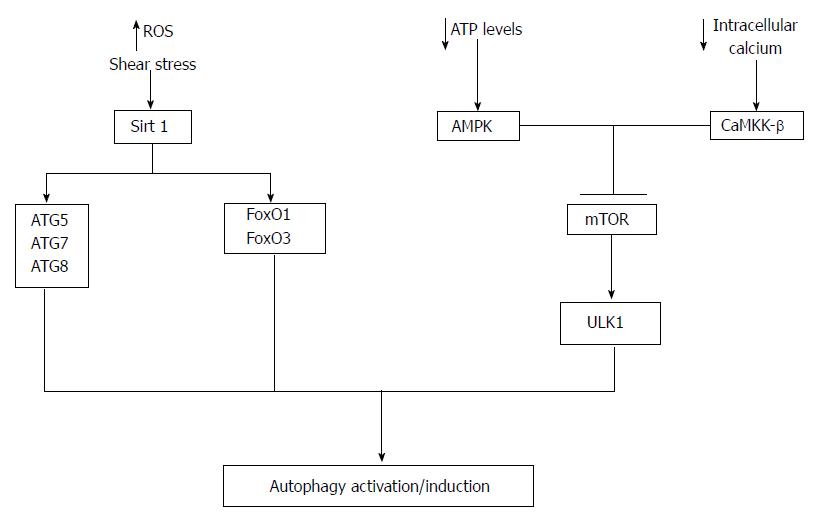
Figure 2 Autophagy activation in endothelial cells.
A number of mechanisms potentially regulate autophagy activation in endothelial cells. A decrease in cellular ATP or reduction in growth factors availability leads to the activation of AMP-activated protein kinase (AMPK). Once activated AMPK can inhibit mTOR leading to the activation of ULK1 and hence autophagy activation. In addition decreases in intracellular calcium can activate CaMKK-β leading to mTOR inhibition and autophagy activation. Moreover, Sirt1 can activate autophagy via deacetylation of ATG5, ATG7, ATG8 and increased transcription of FoxO1 and FoxO3 that then regulate the expression ATGs via deacetylation of Akt. Reactive oxygen species (ROS) and shear stress are important regulators of Sirt1 activity.
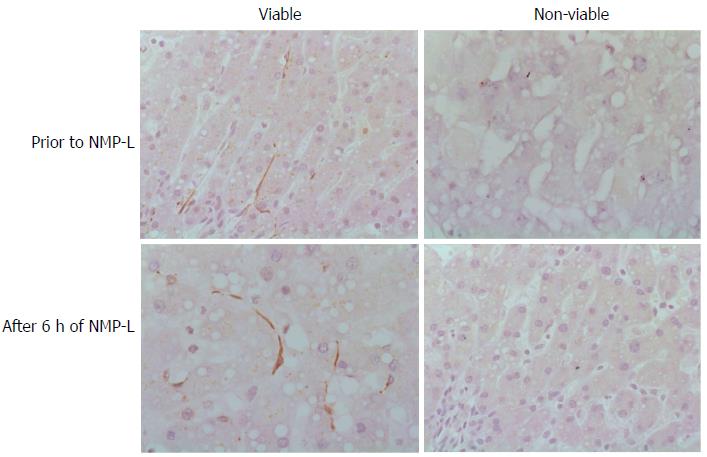
Figure 3 Autophagy and liver viability following normothermic machine liver perfusion.
Immunohistochemical analysis was performed for the autophagy protein LC3B in liver tissue prior to normothermic machine liver perfusion (NMP-L) and after 6 h of NMP-L. Livers deemed viable after NMP-L demonstrated LC3B immunostaining in the hepatic sinusoids prior to commencing NMP-L and at the end of perfusion. Donor livers not fulfilling viability criteria demonstrated no LC3B Immunostaining.
- Citation: Boteon YL, Laing R, Mergental H, Reynolds GM, Mirza DF, Afford SC, Bhogal RH. Mechanisms of autophagy activation in endothelial cell and their targeting during normothermic machine liver perfusion. World J Gastroenterol 2017; 23(48): 8443-8451
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8443.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8443