Copyright
©The Author(s) 2017.
World J Gastroenterol. Dec 7, 2017; 23(45): 8017-8026
Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8017
Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8017
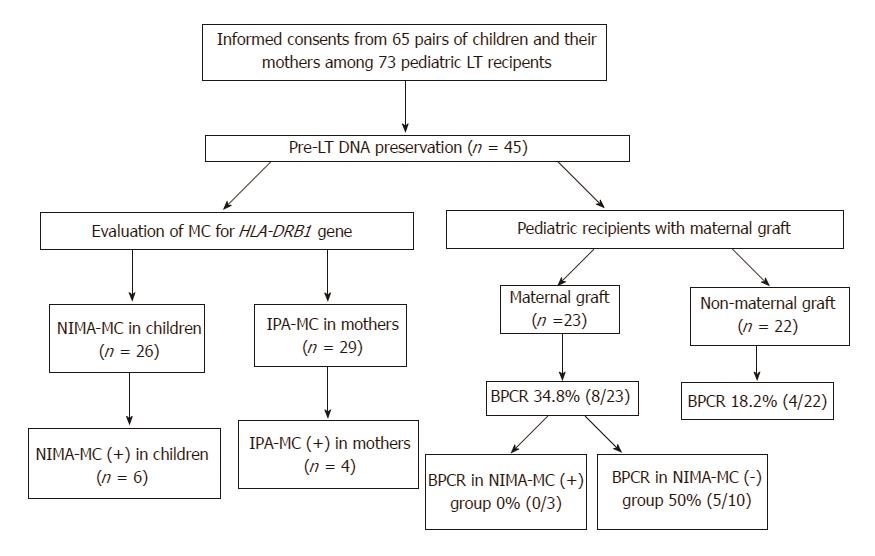
Figure 1 Evaluation of pretransplantation fetal-maternal microchimerism in pediatric recipients of liver transplants and their mothers and the rates of biopsy-proven cellular rejection in various recipient groups.
LT: Liver transplantation; MC: Microchimerism; NIMA: Non-inherited maternal antigen; IPA: Inherited paternal antigen; BPCR: Biopsy-proven cellular rejection.
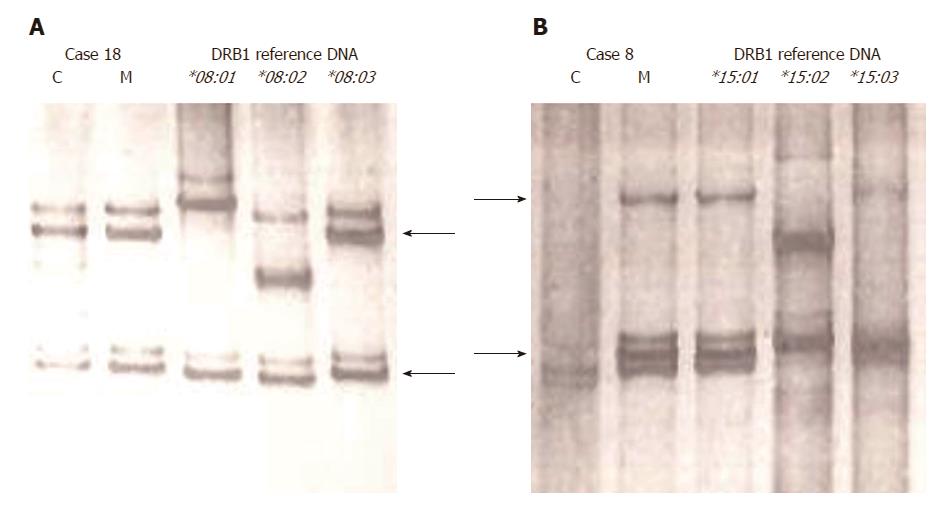
Figure 2 Detection of pretransplantation HLA-DRB1 microchimerism to non-inherited maternal antigen in pediatric recipients of liver transplants, using nested PCR-SSCP method.
A: MC to HLA-DRB1 NIMA (DRB1*08:03) is positive in the child; B: MC to HLA-DRB1 NIMA (DRB1*15:01) is negative in the child. SSCP analyses were performed using nested PCR products in the recipients (children), non-nested PCR products in their mothers, and reference DNA. The SSCP band pattern of DNA of the recipients is compared with that of their mothers (arrows) and corresponding reference DNA (arrows), run in the same gel. C: Child; HLA: Human leukocyte antigen; M: Mother; SSCP: Single-strand conformation polymorphism.
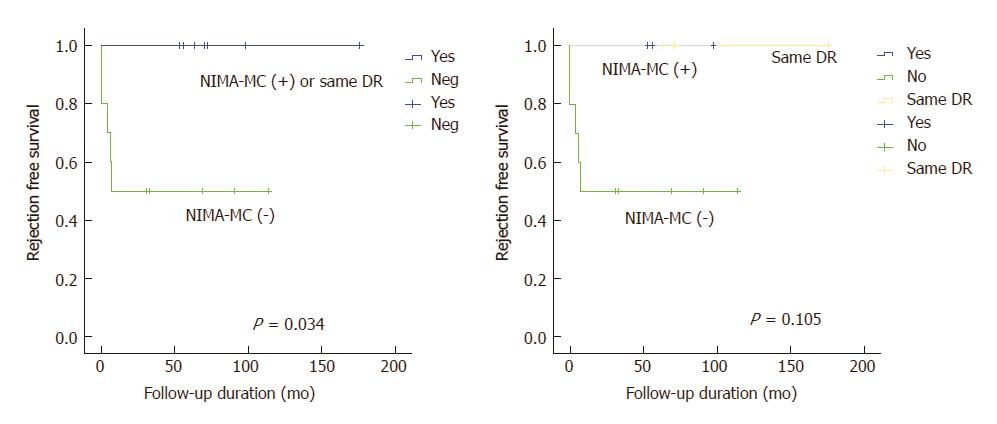
Figure 3 Biopsy-proven cellular rejection-free survival curve according to microchimerism to non-inherited maternal antigen positivity or same HLA DR status to the maternal graft.
BPCR: Biopsy-proven cellular rejection; NIMA-MC: microchimerism (MC) to non-inherited maternal antigen (NIMA).
- Citation: Yi NJ, Park MS, Song EY, Ahn HY, Byun J, Kim H, Hong SK, Yoon K, Kim HS, Ahn SW, Lee HW, Choi Y, Lee KW, Suh KS, Park MH. Pretransplantation fetal-maternal microchimerism in pediatric liver transplantation from mother. World J Gastroenterol 2017; 23(45): 8017-8026
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8017