Copyright
©The Author(s) 2017.
World J Gastroenterol. Sep 7, 2017; 23(33): 6128-6136
Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
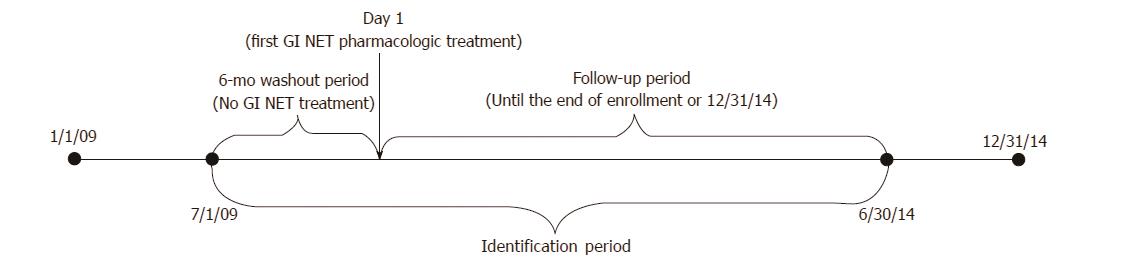
Figure 1 Study timeline.
The first gastrointestinal neuroendocrine tumors (GI NET) pharmacologic treatment claim on or after the appearance of the GI NET diagnosis code and within the ID period (7/1/2009 to 6/30/2014) was considered to be the index date. Patients were required to be enrolled for a baseline period of at least six months before the index date. Patient follow-up was variable and continued until the end of enrollment or the study end date (12/31/14), whichever was first.
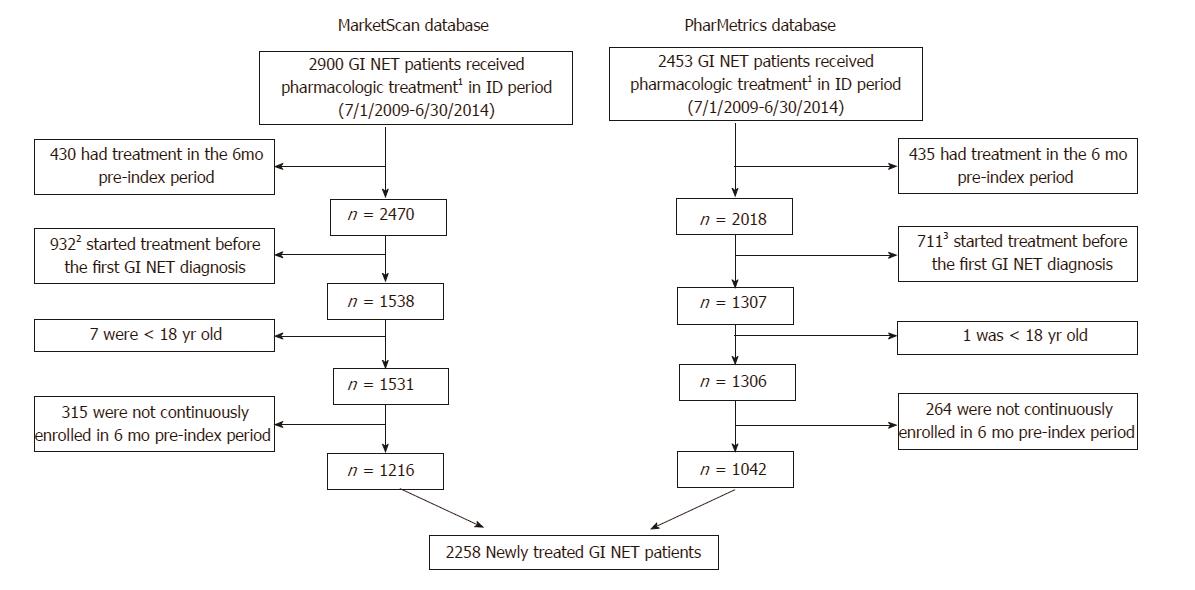
Figure 2 Patient identification.
There were 2900 and 2453 gastrointestinal neuroendocrine tumors (GI NET) patients who also had a claim for pharmacologic treatment between 7/1/2009 and 6/30/2014 in the MarketScan and PharMetrics databases, respectively. After excluding patients who had treatment during a 6-mo pre-index period (and therefore were considered to be continuing, rather than initiating, treatment); received treatment before receiving a diagnosis of GI NET; were < 18 yr old; or were not continuously enrolled in the 6-mo pre-index period, there remained 2258 newly treated GI NET patients who were included in the study. 1Somatostatin analogues (SSAs), targeted therapy, cytotoxic chemotherapy, or interferon; 2324 (34.8%) within 3 mo, and 516 (55.4%) within 6 mo; 3249 (35.0%) within 3 mo, and 380 (53.4%) within 6 mo.
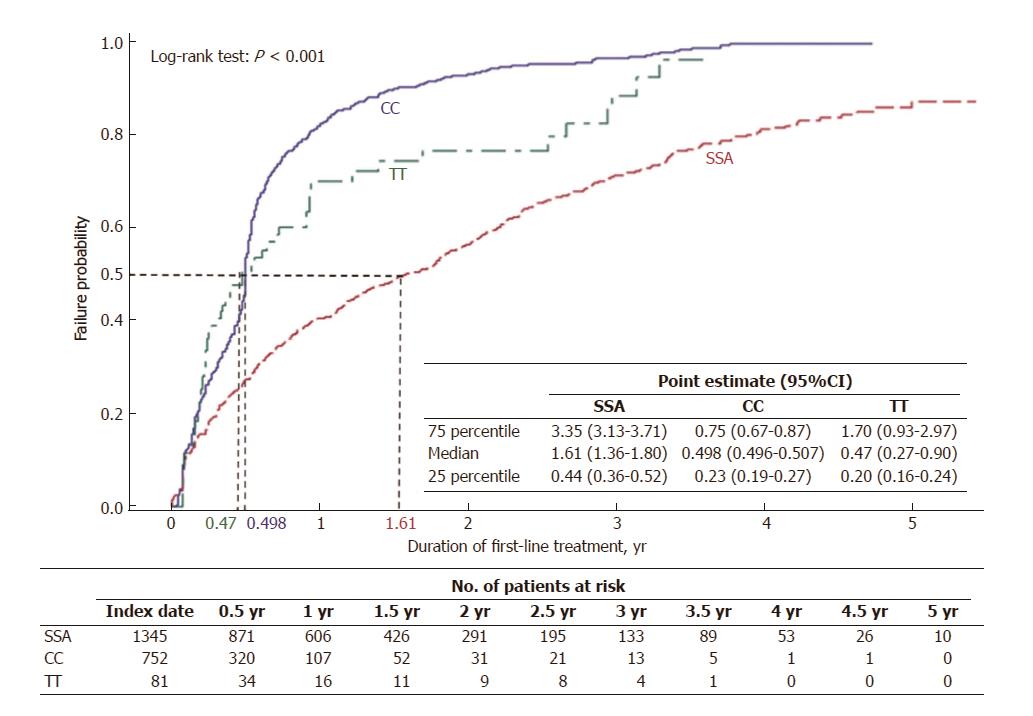
Figure 3 Time to discontinuation of first-line treatment.
By 588 d of treatment (1.61 yr), half of SSA initiators had discontinued treatment, compared to 182 d (0.498 yr) for half of CC users and 171 d (0.47 yr) for half of TT users to discontinue treatment. SSA: Somatostatin analogues; CC: Cytotoxic chemotherapy; TT: Targeted therapy.
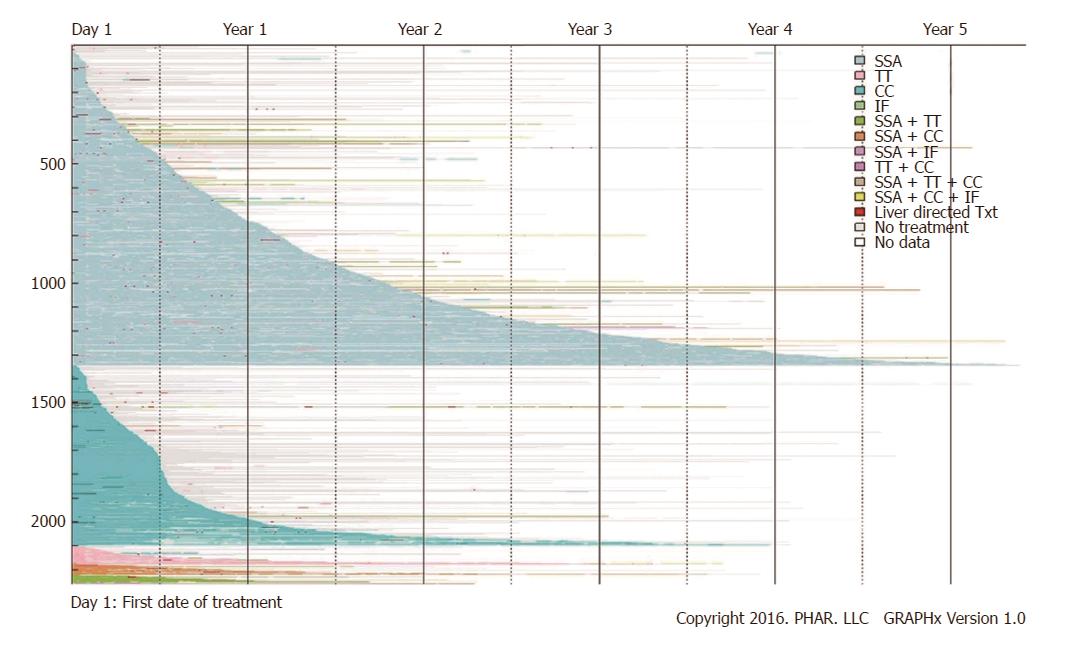
Figure 4 Pharmacologic treatment.
By end of study follow-up [mean (SD, median) of 576 d (447.1, 454)], 58.9% (n = 1331) patients had stopped pharmacologic therapy completely. These patients (no pharmacologic treatment claims, but remained enrolled in one of the databases) can be identified as colored line segments that terminate in gray segments of variable length, with the gray representing the period of no treatment. An additional 32.7% (n = 738) continued their initial therapy until the end of their enrollment; these patients were still receiving their first-line therapy at the time they left a covered plan or reached the end of study. This pattern is shown as a colored segment terminating in white. The remaining 8.4% (n = 189) were observed to change pharmacologic treatment during the follow-up period (a colored segment terminating in different colored segment). Liver directed therapy (short, red segments) appears dispersed throughout periods of both pharmacologic treatment (colored segments) and periods of no pharmacologic treatment (gray segments). SSA: Somatostatin analogues; CC: Cytotoxic chemotherapy; TT: Targeted therapy; IF: Interferon.
- Citation: Benson III AB, Broder MS, Cai B, Chang E, Neary MP, Papoyan E. Real-world treatment patterns of gastrointestinal neuroendocrine tumors: A claims database analysis. World J Gastroenterol 2017; 23(33): 6128-6136
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6128