Copyright
©The Author(s) 2017.
World J Gastroenterol. Mar 21, 2017; 23(11): 1974-1979
Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.1974
Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.1974
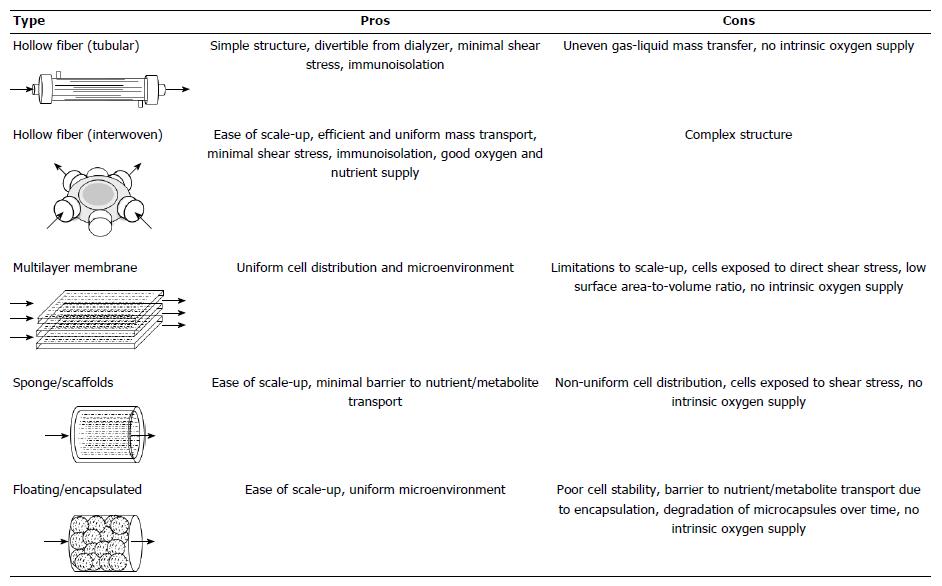
Figure 1 Artificial liver device designs.
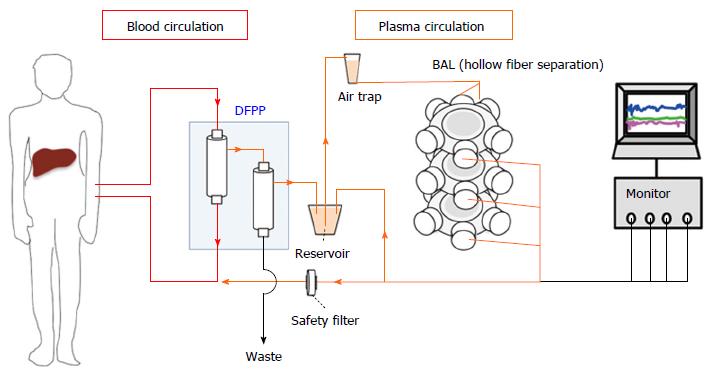
Figure 2 Bioartificial liver system with human pluripotent stem cells-derived hepatic cells using double filtration plasmapheresis.
In a bioartificial liver (BAL) system, patient plasma is first separated from whole blood by double filtration plasmapheresis (DFPP). Plasma then perfuses a bioartificial device using hydrophilic hollow fibers. The human pluripotent stem cells (hPSCs)-derived hepatic cells are inoculated at the outside of the hollow fibers. The detoxified patient plasma is filtered once more before returning to the patient’s blood stream. The hollow fiber membranes and safety filter provide two layers of separation between the patient’s blood stream and the hPSC-derived hepatic cells.
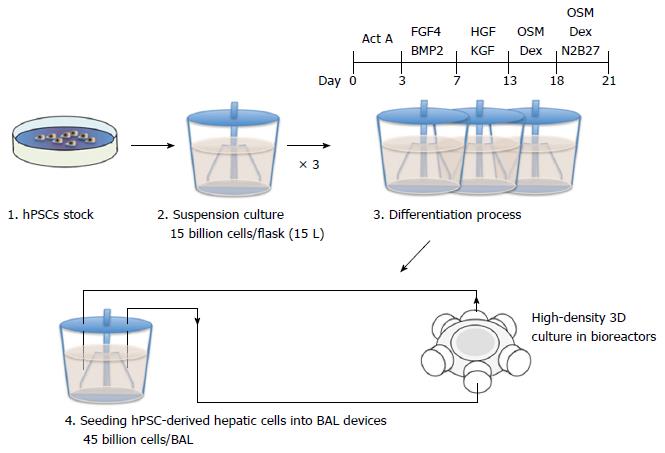
Figure 3 A strategy and cell number estimate of human pluripotent stem cells-derived hepatic cells in the mass production of bioartificial liver devices.
Undifferentiated human pluripotent stem cells (hPSCs) can be expanded in a 15 L suspension culture system up to a maximum of 15 billion cells[37]. Three of these suspension culture flasks will be required to prepare 45 billion cells for a clinical-scale bioartificial liver (BAL) device. After inducing hepatic differentiation, the hPSC-derived hepatic cells will be cultured at high density in bioreactors to generate a BAL device.
- Citation: Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol 2017; 23(11): 1974-1979
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/1974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.1974