Copyright
©The Author(s) 2016.
World J Gastroenterol. Nov 7, 2016; 22(41): 9057-9068
Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9057
Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9057
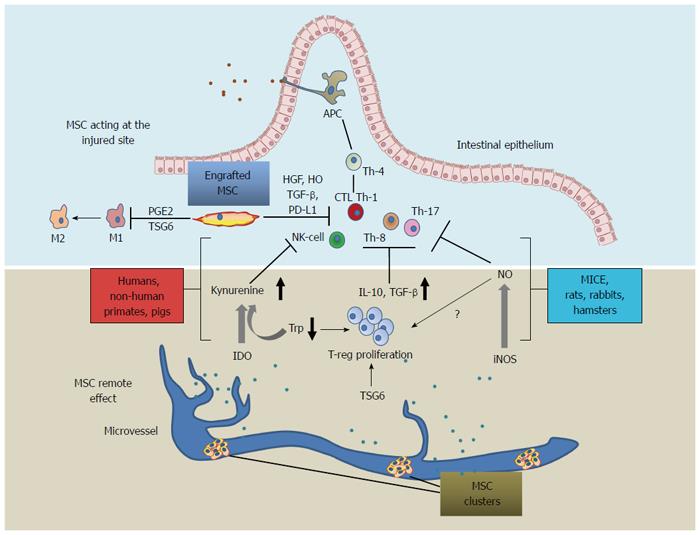
Figure 1 Differences in mesenchymal stromal cell mechanisms of action across species: scheme representing engrafted mesenchymal stromal cell acting at the injured site (upper side) and mesenchymal stromal cell acting at a distance as a reservoir of anti-inflammatory mediators (lower side).
Several MSC-driven mechanisms concur to orientate activated lymphocytes toward the T-regulatory phenotype. TSG-6 enhances proliferation of T-reg lymphocytes and inhibits secretion of pro-inflammatory cytokines by macrophages, the latter undergoing a phenotype shift toward the anti-inflammatory one (from M1 to M2) driven by MSC-secreted PGE2. PD-L1 mediate an anti-inflammatory action by cell-cell contact. MSC immunomodulation, acts primarily through the IDO-mediated mechanism in humans, non-human primates, and pigs; while iNOS acts in mice, rats, hamsters and rabbits, inducing the T-reg proliferation through a mechanism that is still unclear; TSG-6 is a common effector across species which is assumed to mediate both in situ and remote effects. MSC: Mesenchymal stromal cells; TSG6: Tumor necrosis factor-inducible gene 6 protein; IDO: Indoleamine 2,3-dioxygenase; iNOS: Nitric oxide synthase; Trp: Tryptophan; PGE2: Prostaglandin E2; TGF-β: Transforming growth factor-β; PD-L1: Programmed death-ligand 1; HO: Heme-oxigenase-1; HGF: Hepatocyte growth factor; APC: Antigen-presenting cell; T-reg: Regulatory T lymphocytes; macr: Macrophage; M1-M2 macr. shift: Macrophage shift from M1 pro-inflammatory phenotype to M2 anti-inflammatory phenotype.
- Citation: Dothel G, Raschi E, Rimondini R, De Ponti F. Mesenchymal stromal cell-based therapy: Regulatory and translational aspects in gastroenterology. World J Gastroenterol 2016; 22(41): 9057-9068
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9057.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9057