Copyright
©The Author(s) 2016.
World J Gastroenterol. May 21, 2016; 22(19): 4732-4740
Published online May 21, 2016. doi: 10.3748/wjg.v22.i19.4732
Published online May 21, 2016. doi: 10.3748/wjg.v22.i19.4732
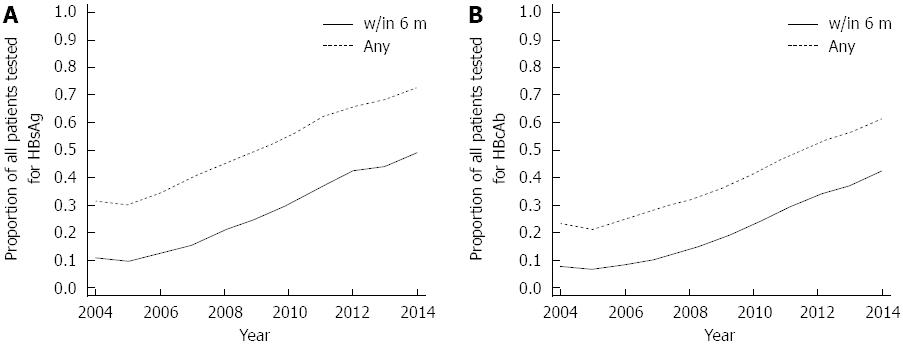
Figure 1 Proportion of all patients with pretreatment hepatitis B surface antigen (A) and hepatitis B core antibody (B) testing over time.
Over the study period, pretreatment HBsAg testing within six months of anti-CD20 Ab initiation increased steadily, in parallel with pretreatment HBsAg testing obtained at any time. Over the study period, pretreatment HBcAb testing within six months of anti-CD20 Ab initiation steadily increased, in parallel with pretreatment HBcAb testing obtained at any prior time. HBsAg: Hepatitis B surface antigen; HBcAb: Hepatitis B core antibody.
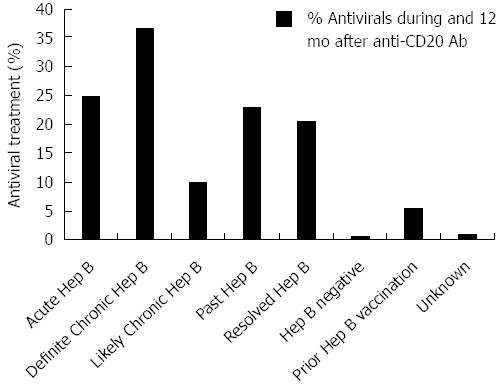
Figure 2 Hepatitis B antiviral treatment by hepatitis B category.
Mean hepatitis B antiviral treatment use during anti-CD20 Ab treatment and 12 mo follow-up is profiled by hepatitis B category throughout the study period.
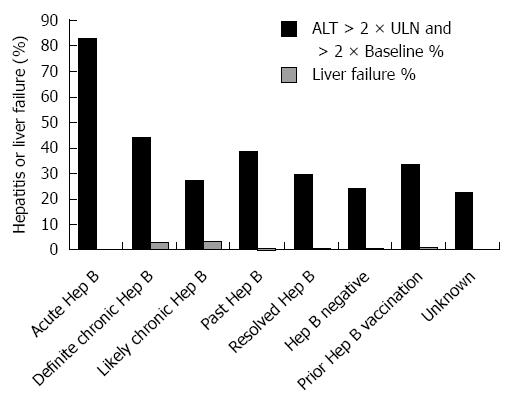
Figure 3 Incidence of hepatitis and liver failure by hepatitis B category.
The incidence of hepatitis and liver failure during anti-CD20 Ab treatment and 12 mo follow-up is profiled by hepatitis B category throughout the study period.
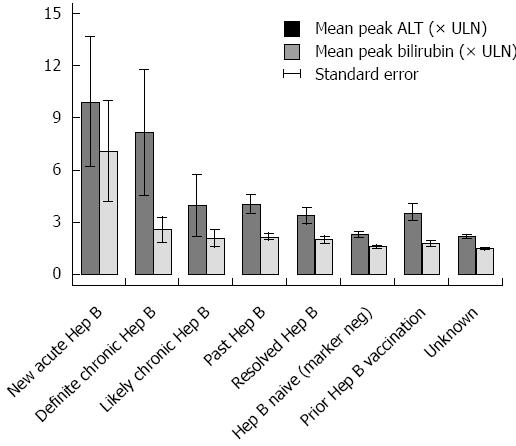
Figure 4 Mean peak ALT and bilirubin by hepatitis B category.
The mean peak ALT and bilirubin of patients during anti-CD20 Ab treatment and 12 mo follow-up is profiled by hepatitis B category throughout the study period.
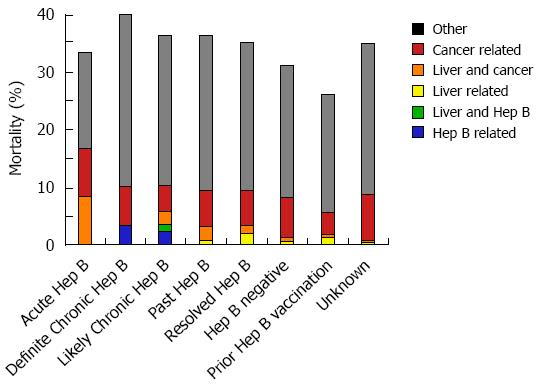
Figure 5 Incidence of overall, hepatitis-B associated, liver-related or cancer-related mortality by hepatitis B category.
The overall, hepatitis-B associated, liver-related or cancer-related mortality incidence during anti-CD20 Ab treatment and 12 mo follow-up is profiled by hepatitis B category throughout the study period.
- Citation: Hunt CM, Beste LA, Lowy E, Suzuki A, Moylan CA, Tillmann HL, Ioannou GN, Lim JK, Kelley MJ, Provenzale D. Veterans health administration hepatitis B testing and treatment with anti-CD20 antibody administration. World J Gastroenterol 2016; 22(19): 4732-4740
- URL: https://www.wjgnet.com/1007-9327/full/v22/i19/4732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i19.4732