Copyright
©The Author(s) 2015.
World J Gastroenterol. Jun 28, 2015; 21(24): 7457-7467
Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7457
Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7457
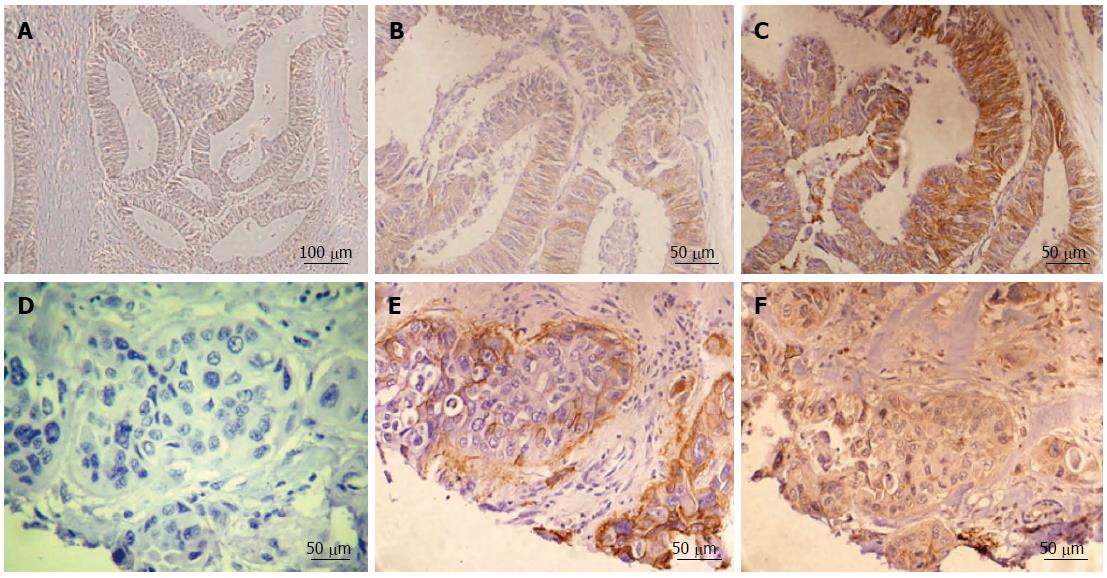
Figure 1 Representative immunohistochemistry examples for both integrin αvβ6 and matrix metalloproteinase-9 in colon cancer.
Negative expression for integrin αvβ6 (A), low integrin αvβ6 expression in the tumor edge portion (B) and high integrin αvβ6 expression in the tumor central area (C). In the paired serial sections, (D) is a negative control. Positive αvβ6 expression in the edge of both invasive tumor islands and tumor budding are shown in (E). Strong matrix metalloproteinase-9 expression (F) was also identified in invasive tumor edge portions. A scale bar is shown in the right lower corner of each figure.
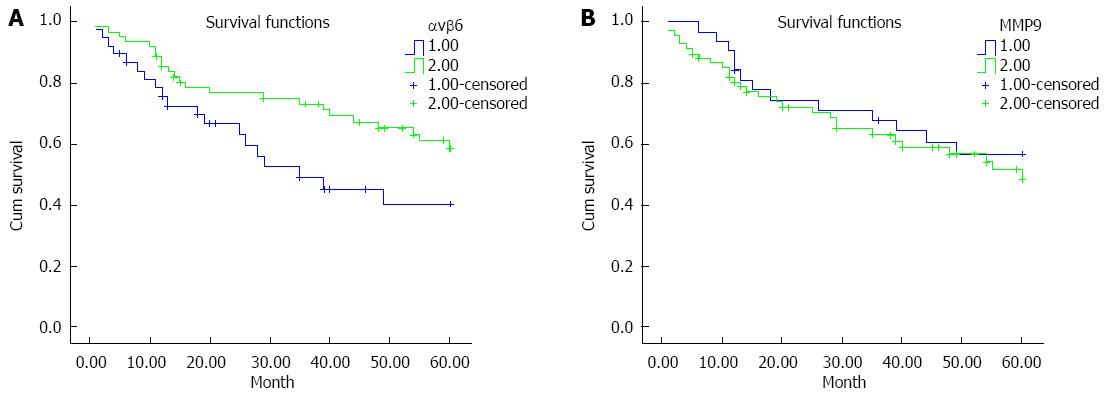
Figure 2 Kaplan-Meier survival analysis using a log-rank test.
The samples were grouped according to integrin αvβ6 (A) or matrix metalloproteinase-9 (MMP-9) (B) expression level (negative or positive) and analyzed using the log-rank test for overall survival.
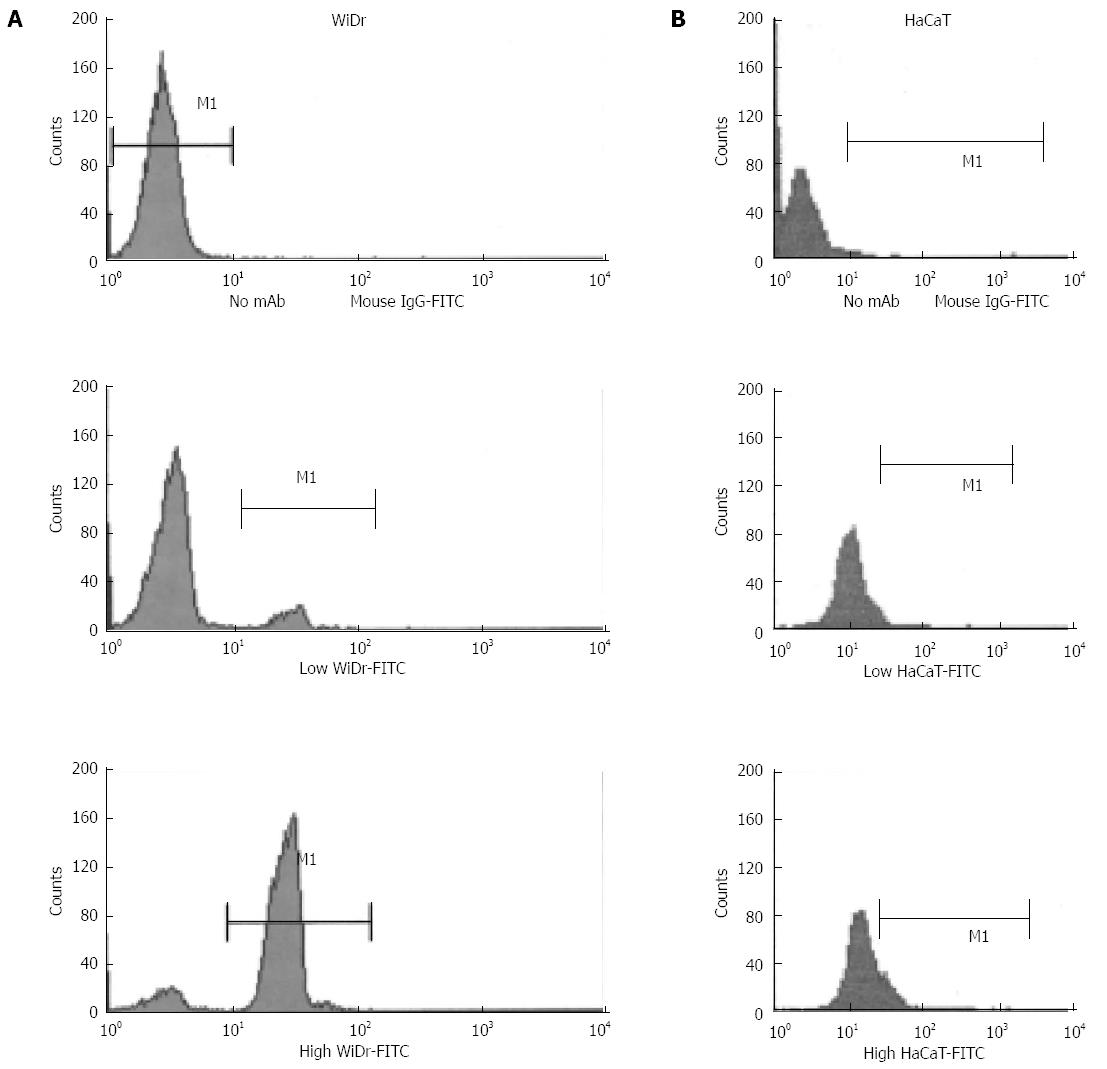
Figure 3 Effects of cell density on integrin αvβ6 in both WiDr and HaCaT cells.
WiDr (A) and HaCaT (B) cells harvested from low- and high-density cultures were analyzed by FACScan for integrin αvβ6 expression. The cells were incubated with either no primary antibody (upper panels) or function-blocking integrin αvβ6 mAb 10D5 (lower panels) and with goat anti-mouse IgG conjugated with FITC. The data are representative of three similar experiments.
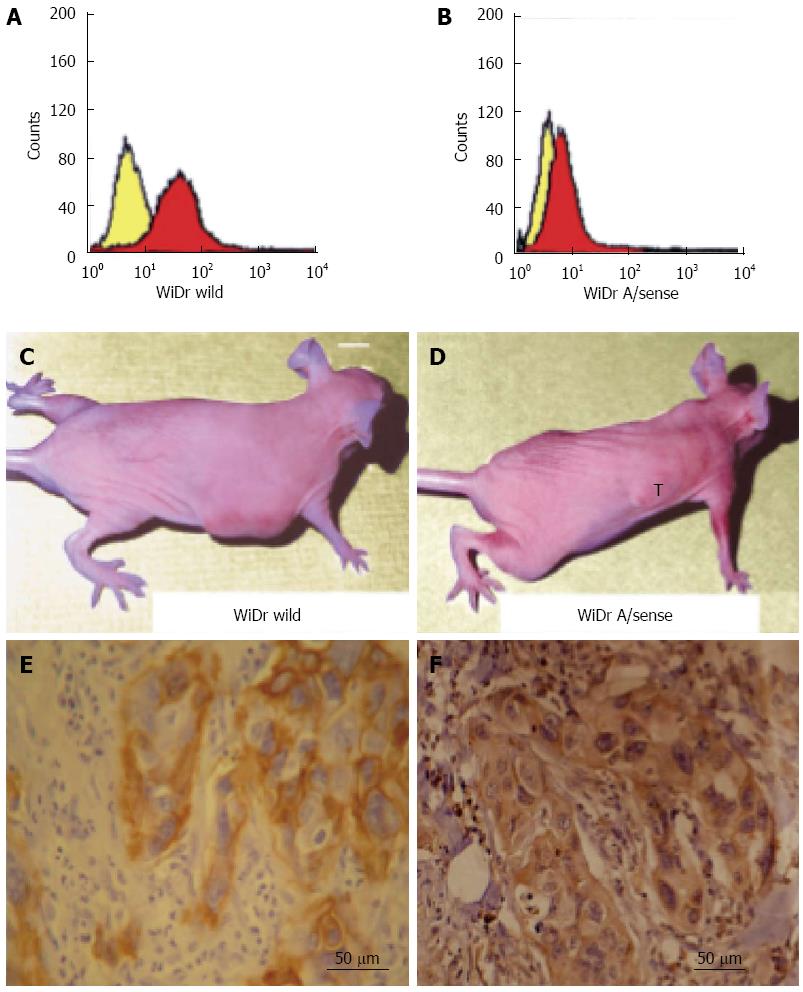
Figure 4 Integrin αvβ6 promotes invasive tumor growth in nude mice.
Wild-type WiDr cells (A) and antisense β6 WiDr transfectants (B) were analyzed by FACScan for the expression of integrin αvβ6. The yellow and red histograms represent analyses in the absence and presence of E7P6 primary antibody; the secondary antibody (goat anti-mouse IgG) was conjugated with phycoerythrin. Tumor invasive growth after 6 wk following subcutaneous inoculation with wild-type WiDr cells (C) or antisense beta 6 WiDr transfectants (D). Increased expression of integrin αvβ6 (E) and matrix metalloproteinase-9 (MMP-9) (F) was identified in serial sections of tumor xenografts inoculated with wild-type WiDr cells. One section was selected from every 10 serial sections. The figures (E) and (F) are the 1st section and 21st section selected. The thickness of each section was 5 μm. A scale bar is shown in the right lower corner of (E) and (F).
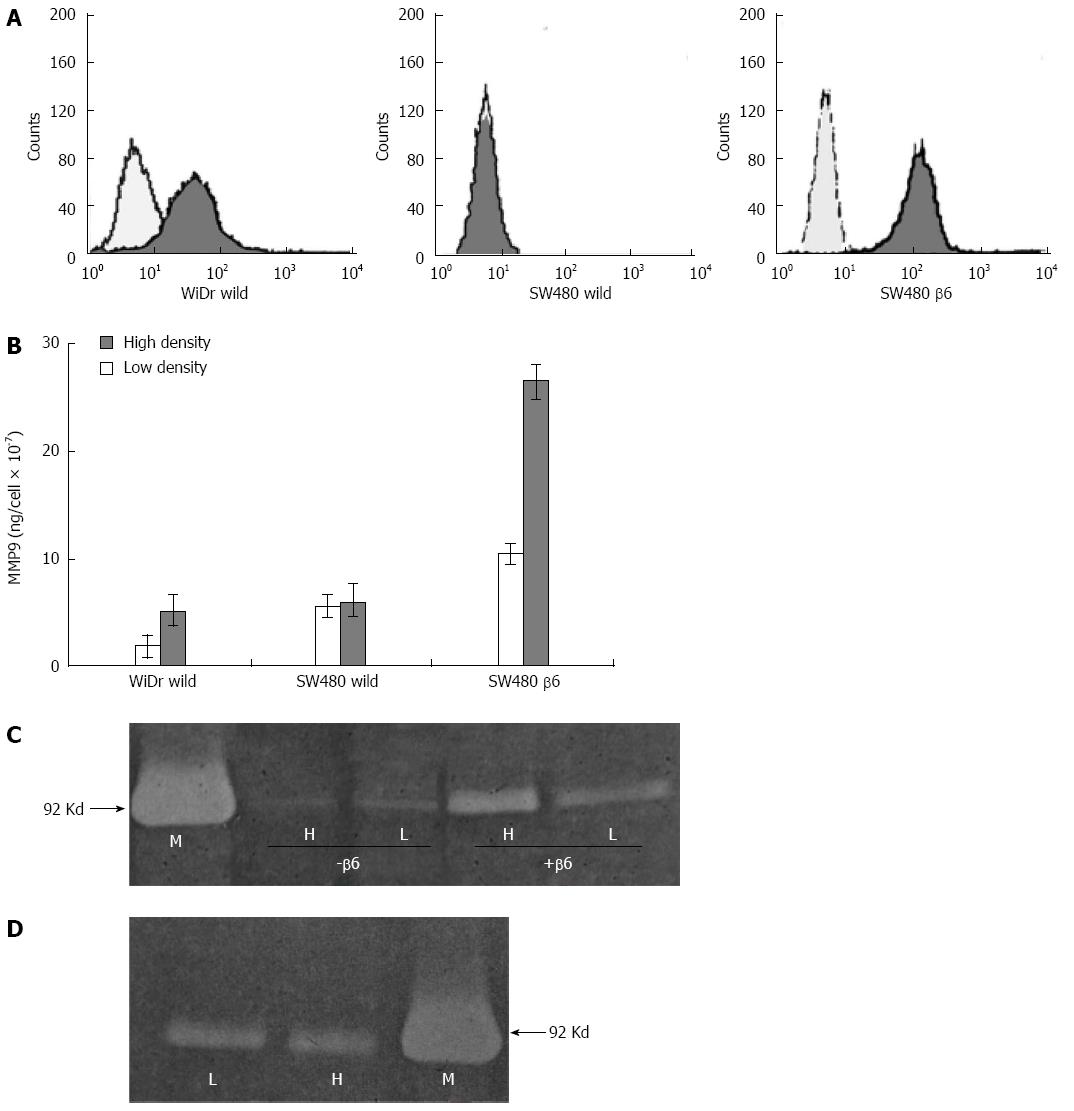
Figure 5 Effects of cell density on matrix metalloproteinase-9 secretion.
A: Wild-type WiDr and SW480 cells and β6 SW480 transfectants were analyzed by FACScan for integrin αvβ6 expression. The pale and black histograms represent analyses in the absence and presence of E7P6 primary antibody; the secondary antibody (goat anti-mouse IgG) was conjugated with phycoerythrin; B: Gelatinase B assay (Biotrak) showing levels of matrix metalloproteinase-9 (MMP-9) in TCM (20-fold concentrated). The data represent the mean MMP-9 levels per cell for three experiments; in each experiment, the TCM samples were tested in duplicate; C: Gelatin zymogram showing gelatinase activity in non-concentrated TCM from β6 SW480 (+β6) and wild-type SW480 cells (-β6) cultured at low (L) and high (H) densities. The position of purified MMP-9 (M) is shown on the left; D: Gelatin zymogram showing gelatinase activity in non-concentrated conditioned medium from HaCaT cells cultured at low (L) and high (H) densities. The position of purified MMP-9 (M) is shown on the right.
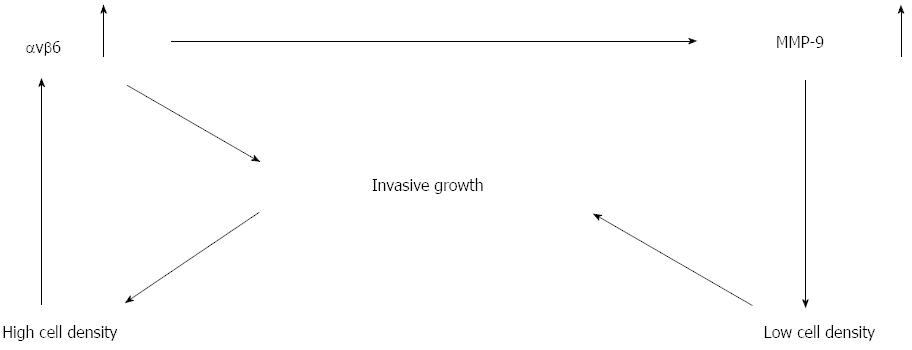
Figure 6 Model of tumor cell infiltrating growth in which integrin ανβ6 provides a self-perpetuating system in colon cancer progression.
ανβ6: Integrin ανβ6; MMP-9: Matrix metalloproteinase-9.
- Citation: Yang GY, Guo S, Dong CY, Wang XQ, Hu BY, Liu YF, Chen YW, Niu J, Dong JH. Integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression. World J Gastroenterol 2015; 21(24): 7457-7467
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7457