Copyright
©The Author(s) 2015.
World J Gastroenterol. Apr 21, 2015; 21(15): 4547-4554
Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4547
Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4547
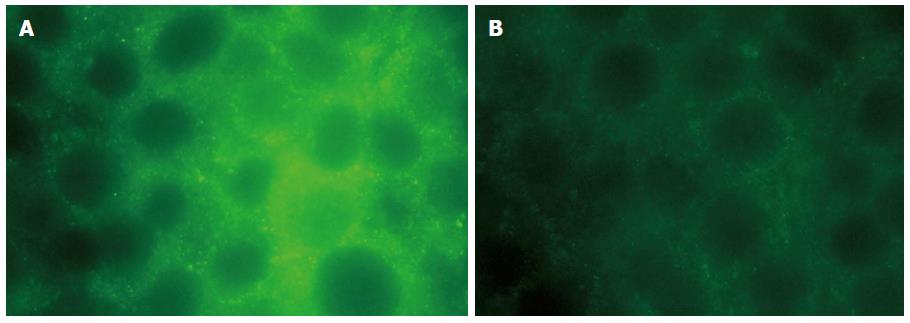
Figure 1 Immunocytochemical localisation of aquaporin 3 in HT-29 cells.
Aquaporin 3 (AQP3) expression in untreated HT-29 cells (left) or cells treated with 20 μg/mL lipopolysaccharide for 12 h (right). Cells were fixed and analysed by immunofluorescence staining for AQP3 protein using FITC-labelled secondary antibody (magnification × 400).
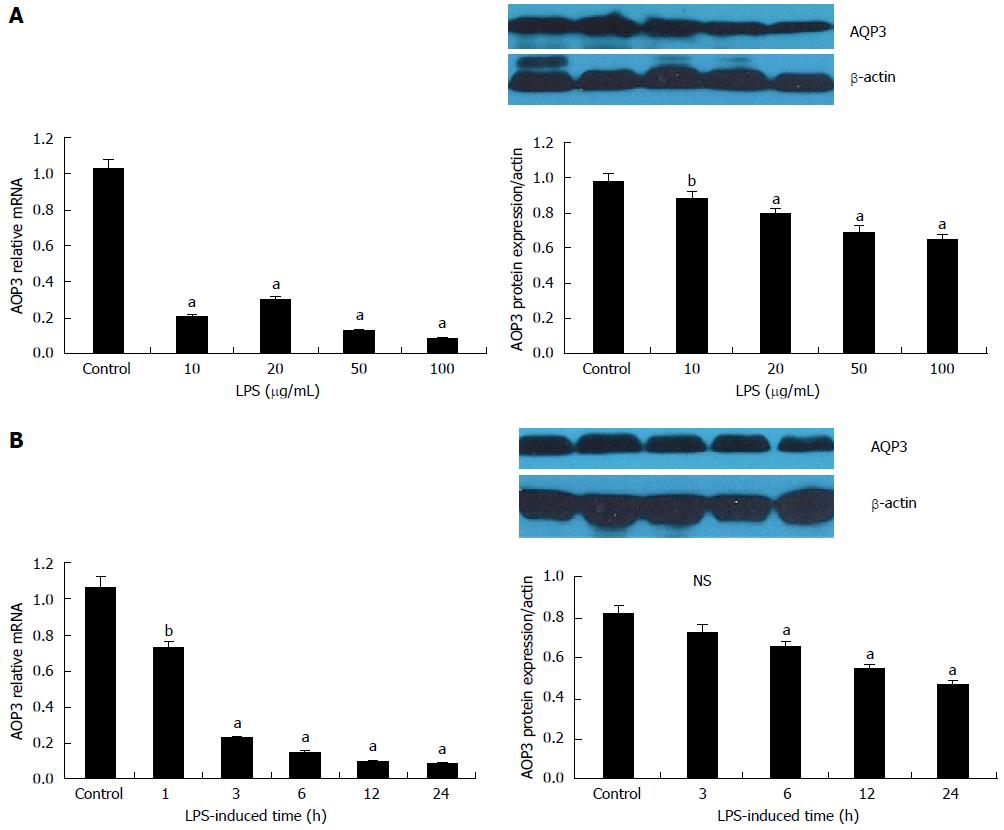
Figure 2 Down-regulation of aquaporin 3 mRNA and protein expression by lipopolysaccharide in HT-29 cells.
A: Dose-dependent decrease in aquaporin 3 (AQP3) mRNA and protein expression by lipopolysaccharide (LPS). Cells were incubated in media supplemented with LPS (0, 10, 20, 50 and 100 μg/mL) for 12 h; B: Time-dependent decrease in AQP3 mRNA and protein expression by LPS. Cells were incubated in media supplemented with 100 μg/mL LPS for various durations (0, 3, 6, 12 and 24 h). The mRNA and protein levels of AQP3 were determined by reverse-transcription PCR and Western blot, respectively. For Western blot, β-actin served as a loading control. Data are presented as mean ± SD of three independent experiments; aP < 0.05, bP < 0.01 vs control; NS: Not significant.
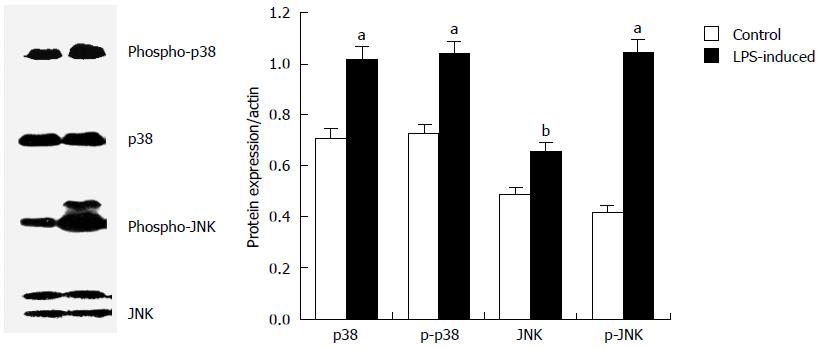
Figure 3 Activation of p38/c-Jun N-terminal kinase by lipopolysaccharide in HT-29 cells.
Cells were treated with 20 μg/mL lipopolysaccharide for 60 min, and protein lysates were prepared and analysed by Western blot. Equal amounts of protein were loaded and probed sequentially with antibodies for phospho-p38 (p-p38), p38 kinase, phospho-c-Jun N-terminal kinase (p-JNK), and JNK. Data represent one of three independent experiments, and quantitative data are presented as mean ± SD from three independent experiments, aP < 0.05, bP < 0.01 vs control.
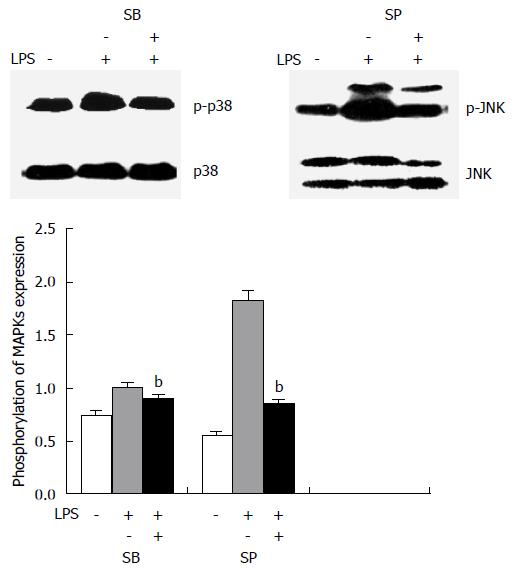
Figure 4 p38/c-Jun N-terminal kinase inhibitors specifically blocked p38/c-Jun N-terminal kinase phosphorylation in HT-29 cells induced by lipopolysaccharide.
Cells were pre-treated with the p38 kinase inhibitor SB203580 (10 μg/mL) or the c-Jun N-terminal kinase (JNK) inhibitor SP600125 (20 μg/mL) for 30 min, before incubation with 20 μg/mL lipopolysaccharide for 60 min. Immunoblot analysis was then performed for phospho-p38 kinase (p-p38), p38 kinase, phospho-JNK (p-JNK), and JNK. bP < 0.01 vs cells without inhibitor treatment.
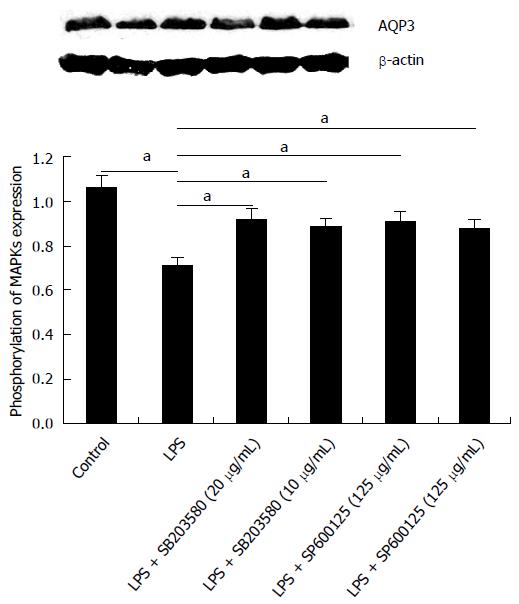
Figure 5 Effect of p38/c-Jun N-terminal kinase inhibitors on lipopolysaccharide-induced down-regulation of aquaporin 3 expression in HT-29 cells.
Cells were incubated with 20 μg/mL lipopolysaccharide for 12 h, after pre-treatment with inhibitors for 30 min. Aquaporin 3 protein expression was determined by Western blot; β-actin served as a loading control. Data represent one of three independent experiments, and quantitative data are presented as mean ± SD from three independent experiments, aP < 0.05 vs control.
-
Citation: Li FX, Huang LZ, Dong C, Wang JP, Wu HJ, Shuang SM. Down-regulation of aquaporin3 expression by lipopolysaccharide
via p38/c-Jun N-terminal kinase signalling pathway in HT-29 human colon epithelial cells. World J Gastroenterol 2015; 21(15): 4547-4554 - URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4547