Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Oct 28, 2014; 20(40): 14778-14786
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14778
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14778
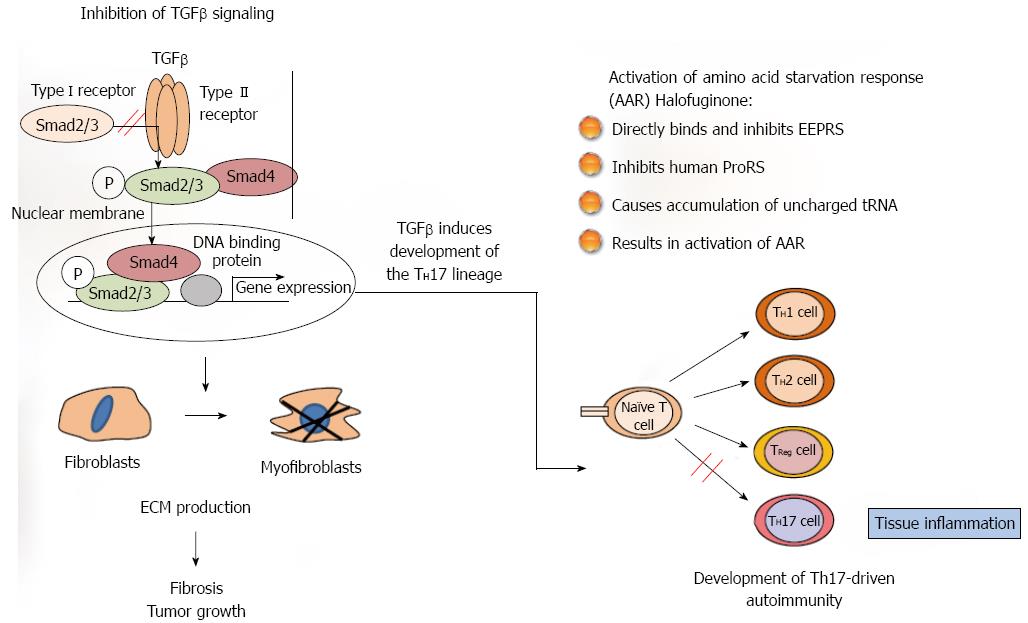
Figure 1 Halofuginone mode of action.
Two pathways have been suggested for the biological activity of halofuginone which may be connected. In fibrosis and in cancer (left side) the tumor growth factor (TGF) β derived from the inflammatory cells binds to the constitutively active type II TGFβ receptor kinase that trans-phosphorylates the type I receptor. Activated type I receptor kinase then phosphorylates Smads 2/3, which subsequently recruit Smad4. The activated Smads 2/3 Smad4 complex then translocates to the nucleus where it regulates specific gene expression. The TGFβ -dependent gene expression causes quiescent fibroblasts differentiation to myofibroblasts, which are responsible for the major increase in matrix synthesis in fibrosis and in tumor stroma which impedes normal function. Halofuginone inhibited TGFβ-dependent Smad3 phosphorylation, causes reduction in fibroblasts differentiation, reduction in the levels of extracellular matrix (ECM) proteins and inhibition of fibrosis and tumor growth. Recently, a second mechanism has been suggested for the halofuginone- dependent inhibition of autoimmune diseases which involves selective prevention of the development of Th17 cells by activating the amino acid starvation response (AAR) - the amino acid starvation response (right side). Naïve CD4+ T cells differentiate into diverse effector and regulatory subsets to coordinate immunity to pathogens while establishing peripheral tolerance. Besides TH1 and TH2 effector subsets, naïve T cells can differentiate into proinflammatory T helper 17 (TH17) cells. These cells are key regulators of autoimmune inflammation. The mechanism by which halofuginone inhibits the AAR is by binding to glutamyl-prolyl-tRNA synthetase (EPRS) and inhibiting prolyl-tRNA synthetase activity (ProRS) causing intracellular accumulation of uncharged tRNA and mimicking reduced cellular proline availability. AAR activation selectively inhibits TH17 differentiation. Thus, halofuginone could potentially be used to address any autoimmune or inflammatory disease associated with Th17 cells. It should be noted (middle) that TGFβ is required for facilitation of differentiation of the inflammatory Th17 cell subset which suggests the existence of a link between the TGFβ and AAR pathways.
- Citation: Pines M. Halofuginone for fibrosis, regeneration and cancer in the gastrointestinal tract. World J Gastroenterol 2014; 20(40): 14778-14786
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14778