Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Oct 28, 2014; 20(40): 14747-14759
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14747
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14747
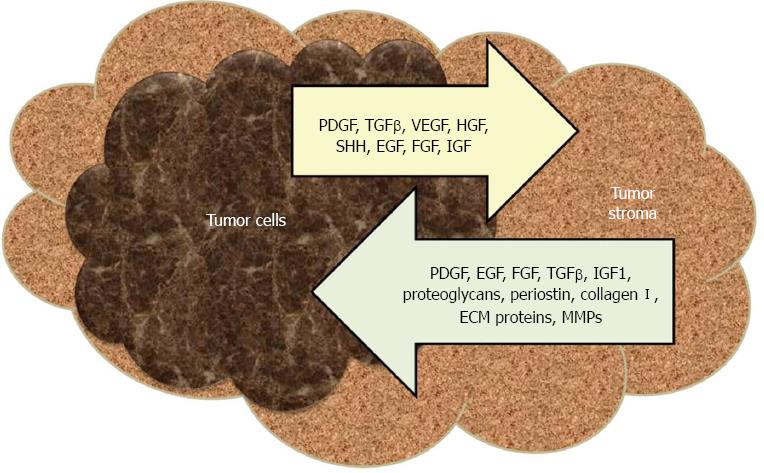
Figure 1 Critical interplay between the pancreatic cancer cells and the microenvironment.
TGF-β: Transforming growth factor-beta; VEGF: Vascular endothelial growth factor; HGF: Hepatocyte growth factor; SHH: Sonic hedgehog; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; IGF: Insulin-like growth factor; MMP: Matrix metalloprotease; PDGF: Platelet derived growth factor.
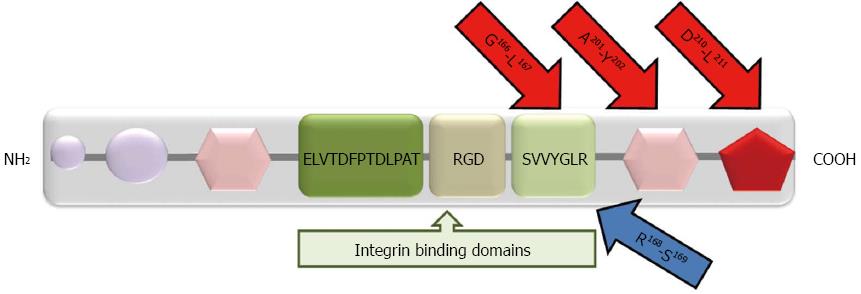
Figure 2 Structural domains of osteopontin.
Purple circles: Matrix binding domains; pink hexagons: Calcium binding sites; Red pentagon: Heparin binding site. There are three integrin binding sequences: (1) Arginine-glycine-aspartic acid (RGD); (2) Serine-valine-valine-tyrosine-glutamate-leucine-arginine (SVVYGLR); and (3) ELVTDFPTDLPAT. MMP cleavage sites (G166-L167; A201-Y202; D210-L211) are shown by red arrows. The thrombin cleavage site (R168-S169) is shown by blue arrow.
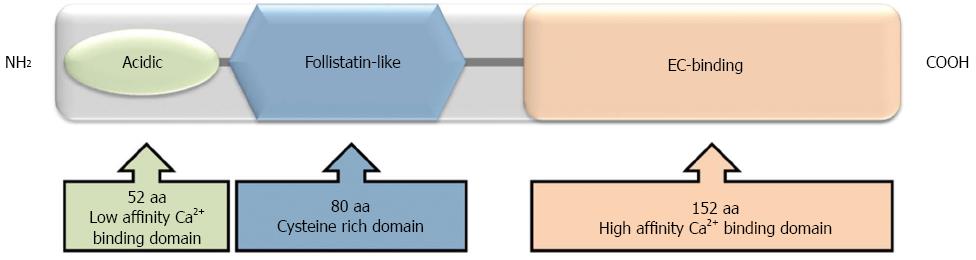
Figure 3 Structural domains of osteonectin.
The N-terminal is a highly acidic, calcium binding domain (low affinity). The follistatin-like domain is rich in cysteine residues. The C-terminal is an extracellular calcium-binding domain (high affinity).
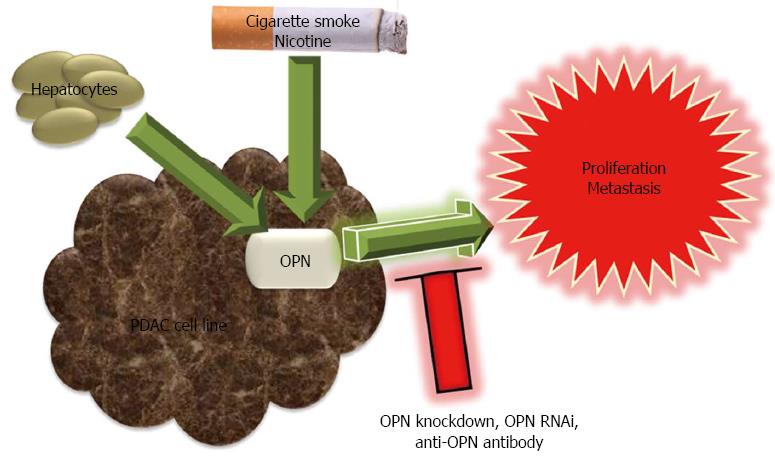
Figure 4 Protumorigenic role of osteopontin in pancreatic cancer development and progression.
Osteopontin (OPN) expression in pancreatic cancer cell lines is associated with increased in vitro proliferation and enhanced growth and metastasis in vivo, which are reversed by OPN knockdown, OPN RNAi and anti-OPN antibody. Exposure to cigarette smoke (including nicotine) and hepatocytes induce OPN expression.
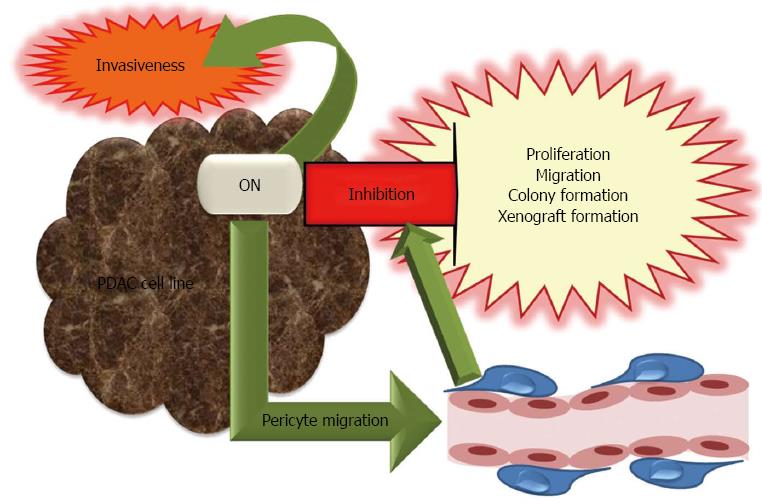
Figure 5 Pro- and anti-tumorigenic roles of osteonectin in pancreatic cancer development and progression.
Osteonectin (ON) is a multifaceted protein with controversial functions of its structural domains. ON is anti-tumorigenic and inhibits proliferation, migration, colony and xenograft formation as well as invasiveness. Facilitation of pericyte migration by ON contributes to inhibition of tumor spread. However, ON is also pro-tumorigenic and induces invasiveness.
- Citation: Kaleağasıoğlu F, Berger MR. SIBLINGs and SPARC families: Their emerging roles in pancreatic cancer. World J Gastroenterol 2014; 20(40): 14747-14759
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14747.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14747